Abstract
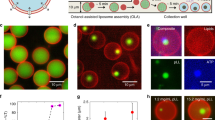
The low membrane permeability of lipophilic drugs was resolved using liposomes as a solubilizing agent and the precise size control of them is a significant parameter in drug carrier technology. Here, we have established a microfluidic octanol-assisted liposome assembly method to produce a surfactant-assisted liposome which has merged by the cytoskeleton drug (Taxotere) encapsulation in a single process step, then a complete microfluidic cellular analysis was performed in trapping cell device with an optofluidic assay for quantifying drug permeability. The optimization of process variables resulted in the formation of liposomes with particle size 6.75 ± 0.5 µm and monodispersity 6.2%, representing encapsulation efficiency and loading capacity of 65.49 ± 3.08% and 10.16 ± 0.32%, respectively. Qualitative and quantitative studies of cellular uptake in MCF-7 cell line that was cultured in the cell trapping chip indicated a significant increase in cellular uptake of carboxyfluorescein-loaded liposomes, suggesting endocytic mechanisms. The drug-loaded liposomes with an IC50 value of 0.55 ± 0.04 μg mL−1 have shown a higher level of cellular inhibition and apoptosis in cells than free Taxotere (2.48 ± 0.01). Furthermore, real-time analysis of the dynamic labeling assay for live/dead cells was investigated. Our data revealed that lab-on-a-chip platforms for the time-lapse fluorescence imaging were applied for drug screening routines.
Graphic abstract
Cytoskeleton drugs encapsulated in liposome using microfluidic approaches and investigation of programmed cell death assay.









Similar content being viewed by others
References
Akhtartavan S, Karimi M, Karimian K, Azarpira N, Khatami M, Heli H (2019) Evaluation of a self-nanoemulsifying docetaxel delivery system. Biomed Pharmacother 109:2427–2433
Becucci L et al (2015) Interaction study of phospholipid membranes with an N-glucosylated β-turn peptide structure detecting autoantibodies biomarkers of multiple sclerosis. Membranes 5:576–596
Bobone S et al (2016) Activity and selectivity of host defense peptides: a complex interplay of multiple equilibria. In: Journal of Peptide Science, 2016. Wiley 111 River ST, Hoboken 07030-5774, NJ USA, pp S36–S36
Bozyczko-Coyne D, McKenna B, Connors T, Neff N (1993) A rapid fluorometric assay to measure neuronal survival in vitro. J Neurosci Methods 50:205–216
Bruus H (2008) Theoretical microfluidics, vol 18. Oxford University Press, Oxford
Chaudhari KR et al (2012) Bone metastasis targeting: a novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J Controll Rel 158:470–478
Chen H, Kim S, Li L, Wang S, Park K, Cheng J-X (2008) Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc Natl Acad Sci 105:6596–6601
Cortes JE, Pazdur R (1995) Docetaxel. J Clin Oncol 13:2643–2655
Cummings BS, Wills LP, Schnellmann RG (2012) Measurement of cell death in Mammalian cells. Curr Protoc Pharmacol 56:12.18. 11–12.18. 24
Deng N-N, Mou C-L, Wang W, Ju X-J, Xie R, Chu L-Y (2014) Multiple emulsion formation from controllable drop pairs in microfluidics. Microfluid Nanofluid 17:967–972
Deng N-N, Yelleswarapu M, Huck WT (2016) Monodisperse uni-and multicompartment liposomes. J Am Chem Soc 138:7584–7591
Deshpande S, Dekker C (2018) On-chip microfluidic production of cell-sized liposomes. Nat Protoc 13:856
Deshpande S, Caspi Y, Meijering AE, Dekker C (2016) Octanol-assisted liposome assembly on chip. Nat Commun 7:10447. https://doi.org/10.1038/ncomms10447
Deshpande S, Birnie A, Dekker C (2017) On-chip density-based purification of liposomes. Biomicrofluidics 11:034106
Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D (1999) Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev 51:691–744
Ducat E, Evrard B, Peulen O, Piel G (2011) Cellular uptake of liposomes monitored by confocal microscopy and flow cytometry. J Drug Deliv Sci Technol 21:469–477
Edwards KA, Bolduc OR, Baeumner AJ (2012) Miniaturized bioanalytical systems: enhanced performance through liposomes. Curr Opin Chem Biol 16:444–452
He T, Liang Q, Zhang K, Mu X, Luo T, Wang Y, Luo G (2011) A modified microfluidic chip for fabrication of paclitaxel-loaded poly (l-lactic acid) microspheres. Microfluid Nanofluid 10:1289–1298
Hood RR, DeVoe DL (2015) High-throughput continuous flow production of nanoscale liposomes by microfluidic vertical flow focusing. Small 11:5790–5799
Hood R, Vreeland W, DeVoe DL (2014) Microfluidic remote loading for rapid single-step liposomal drug preparation. Lab Chip 14:3359–3367
Immordino ML, Brusa P, Arpicco S, Stella B, Dosio F, Cattel L (2003) Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing docetaxel. J Controll Rel 91:417–429
Kastner E, Verma V, Lowry D, Perrie Y (2015) Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int J Pharm 485:122–130
Kazayama Y, Teshima T, Osaki T, Takeuchi S, Toyota T (2015) Integrated microfluidic system for size-based selection and trapping of giant vesicles. Anal Chem 88:1111–1116
Kong F, Zhang X, Hai M (2014) Microfluidics fabrication of monodisperse biocompatible phospholipid vesicles for encapsulation and delivery of hydrophilic drug or active compound. Langmuir 30:3905–3912
Kuramoto H et al (2008) On-chip fabrication of mutifunctional envelope-type nanodevices for gene delivery. Anal Bioanal Chem 391:2729–2733
Kushwah V, Jain DK, Agrawal AK, Jain S (2018) Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf B 172:213–223
Loos WJ, Baker SD, Verweij J, Boonstra JG, Sparreboom A (2003) Clinical pharmacokinetics of unbound docetaxel: role of polysorbate 80 and serum proteins. Clin Pharmacol Ther 74:364–371
Malam Y, Loizidou M, Seifalian AM (2009) Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 30:592–599
Matosevic S, Paegel BM (2011) Stepwise synthesis of giant unilamellar vesicles on a microfluidic assembly line. J Am Chem Soc 133:2798–2800
Mijajlovic M, Wright D, Zivkovic V, Bi J, Biggs MJ (2013) Microfluidic hydrodynamic focusing based synthesis of POPC liposomes for model biological systems. Colloids Surf B 104:276–281
Mohammadi SS, Vaezi Z, Shojaedin-Givi B, Naderi-Manesh H (2019) Chemiluminescent liposomes as a theranostic carrier for detection of tumor cells under oxidative stress. Anal Chim Acta 1059:113–123
Muthu MS, Kulkarni SA, Xiong J, Feng S-S (2011) Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int J Pharm 421:332–340
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybernet 9:62–66
Papahadjopoulos D, Poste G, Schaeffer B (1973) Fusion of mammalian cells by unilamellar lipid vesicles: influence of lipid surface charge, fluidity and cholesterol. BBA Biomembr 323:23–42
Prasad TK, Rangaraj N, Rao NM (2005) Quantitative aspects of endocytic activity in lipid-mediated transfections. FEBS Lett 579:2635–2642
Rezaei N, Mehrnejad F, Vaezi Z, Sedghi M, Asghari SM, Naderi-Manesh H (2020) Encapsulation of an endostatin peptide in liposomes: stability, release, and cytotoxicity study. Colloids Surf B Biointerfaces 185:110552
Sajeesh P, Sen AK (2014) Particle separation and sorting in microfluidic devices: a review. Microfluid Nanofluid 17:1–52
Shum HC, Lee D, Yoon I, Kodger T, Weitz DA (2008) Double emulsion templated monodisperse phospholipid vesicles. Langmuir 24:7651–7653
Sims CE, Allbritton NL (2007) Analysis of single mammalian cells on-chip. Lab Chip 7:423–440
Smallwood I (1996) Handbook of organic solvent properties (Arnold, London) Smallwood handbook of organic solvent properties 1996
Sohail MF et al (2018) Advancements in the oral delivery of Docetaxel: challenges, current state-of-the-art and future trends. Int J Nanomed 13:3145
Stella L et al (2016) The role of thermodynamics in the activity and selectivity of antimicrobial peptides. Biophys J 110:75a–76a
Teh S-Y, Khnouf R, Fan H, Lee AP (2011) Stable, biocompatible lipid vesicle generation by solvent extraction-based droplet microfluidics. Biomicrofluidics 5:044113
Vaezi Z et al (2019) Aggregation determines the selectivity of membrane-active anticancer and antimicrobial peptides: the case of killerFLIP. BBA Biomembr 1862(2):183107
Van Swaay D (2013) Microfluidic methods for forming liposomes. Lab Chip 13:752–767
Walde P, Cosentino K, Engel H, Stano P (2010) Giant vesicles: preparations and applications. ChemBioChem 11:848–865
Wilkemeyer MF, Sebastian AB, Smith SA, Charness ME (2000) Antagonists of alcohol inhibition of cell adhesion. Proc Natl Acad Sci 97:3690–3695
Wlodkowic D, Cooper JM (2010) Tumors on chips: oncology meets microfluidics. Curr Opin Chem Biol 14:556–567
Wlodkowic D, Skommer J, McGuinness D, Faley S, Kolch W, Darzynkiewicz Z, Cooper JM (2009) Chip-based dynamic real-time quantification of drug-induced cytotoxicity in human tumor cells. Anal Chem 81:6952–6959
Wlodkowic D, Khoshmanesh K, Sharpe JC, Darzynkiewicz Z, Cooper JM (2011) Apoptosis goes on a chip: advances in the microfluidic analysis of programmed cell death. Anal Chem 83:6439–6446
Wolbers F, Andersson H, Van den Berg A, Vermes I (2004) Apoptosis induced kinetic changes in autofluorescence of cultured HL60 cells-possible application for single cell analysis on chip. Apoptosis 9:749–755
Xia A et al (2019) A fixed cytometer chip for identification of cell populations and real-time monitoring of single-cell apoptosis under gradient UV radiation. Microfluid Nanofluid 23:78
Zhang T et al (2017) Polysialic acid and Pluronic F127 mixed polymeric micelles of docetaxel as new approach for enhanced antitumor efficacy. Drug Dev Ind Pharm 43:1827–1835
Zhao L, Cao J-T, Wu Z-Q, Li J-X, Zhu J-J (2013) Lab-on-a-Chip for anticancer drug screening using quantum dots probe based apoptosis assay. J Biomed Nanotechnol 9:348–356
Acknowledgements
We are very thankful to Prof. Alireza Ghassempour and Prof. Peyman Salehi (medicinal plants and drugs research institute, Shahid Beheshti University) for kindly providing us with the purified Taxotere. The authors would like to kindly acknowledge all the supports and funding from the University of Tarbiat Modares (Grant IG-39708).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (AVI 88720 kb)
Supplementary material 3 (AVI 7535 kb)
Supplementary material 4 (MP4 10816 kb)
Supplementary material 5 (MP4 2354 kb)
Rights and permissions
About this article
Cite this article
Vaezi, Z., Sedghi, M., Ghorbani, M. et al. Investigation of the programmed cell death by encapsulated cytoskeleton drug liposomes using a microfluidic platform. Microfluid Nanofluid 24, 48 (2020). https://doi.org/10.1007/s10404-020-02353-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-020-02353-3