Abstract
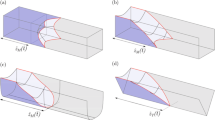
The present work deals with the microfluidic evolution of capillary surfaces that are formed during the priming of microcavity structures with a non-wetting liquid. Due to the large contact angle of the priming liquid, a trapping of air within the microcavities poses a major impediment to a complete filling. We tackle this issue by developing a two-dimensional analytical model describing the geometrical shape of capillary surfaces formed in microcavity structures. In particular, the model is employed to derive two quantitative conditions for a void-free priming of a microcavity structure in terms of its aspect ratio, rounding parameters and the channel width. Microfluidic experiments are performed to verify the analytical results. Finally, we make use of the model to demonstrate a pressure-driven aliquoting of a non-wetting sample liquid in a flow chamber with an array of 55 microcavities by introducing a second immiscible liquid acting as a sealant. In this way, our work constitutes a basis for the design of microcavity-based liquid aliquoting structures that are used in various fields of application like PCR arrays, cell culture chips or digital reaction arrays.





Similar content being viewed by others
References
Chibbaro S, Costa E, Dimitrov DI, Diotallevi F, Milchev A, Palmieri D, Pontrelli G, Succi S (2009) Capillary filling in microchannels with wall corrugations—a comparative study of the Concus-Finn criterion by continuum, kinetic and atomistic approaches. Langmuir 25(21):12653–12660
Goldschmidtboeing F, Rabold M, Woias P (2006) Strategies for void-free liquid filling of micro cavities. J Micromech Microeng 16:1321–1330
Haeberle S, Zengerle R (2007) Microfluidic platforms for lab-on-chip applications. Lab Chip 7(9):1094–1110
Heyries K et al (2011) Megapixel digital PCR. Nat Methods 8:649–651
Hoffmann J, Trotter M, von Stetten F, Zemgerle R, Roth G (2012) Solid-phase PCR in a picowell array for immobilizing and arraying 100,000 PCR products to a microscope slide. Lab Chip 12:3049–3054
Hung TQ, Sun Y, Poulsen CE, Linh-Quyen T, Chin WH, Bang DD, Wolff A (2015) Miniaturization of a micro-optics array for highly sensitive and parallel detection on an injection moulded lab-on-a-chip. Lab Chip 15:2445–2451
Jang M, Park CK, Lee NY (2014) Modification of polycarbonate with hydrophilic/hydrophobic coatings for the fabrication of microdevices. Sens Actuators B 193:599–607
Jensen MJ, Goranovic G, Bruus H (2004) The clogging pressure of bubbles in hydrophilic microchannel contractions. J Micromech Microeng 14:876–883
Lindstrom S, Hammond M, Brismar H, Andersson-Svahn H, Ahmadian A (2009) PCR amplification and genetic analysis in a microwell cell culturing chip. Lab Chip 9:3465–3471
Liu H-B, Ramalingam N, Jiang Y, Dai C-C, Hui KM, Gong H-Q (2009) Rapid distribution of a liquid column into a matrix of nanoliter wells for parallel real-time quantitative PCR. Sens Actuators B 135:671–677
Margulies M et al (2005) Genome sequencing in microfaricated high-density picolitre reactors. Nature 437:376–380
Mark D, Weber P, Lutz S, Focke M, Zengerle R, von Stetten F (2011) Aliquoting on the centrifugal microfluidic platform based on centrifugo-pneumatic valves. Microfluid Nanofluid 10:1279–1288
Matsubara Y, Kerman K, Kobayashi M, Yamamura S, Morita Y, Takamura Y, Tamiya E (2004) On-chip nanoliter-volume multiplex TaqMan polymerase chain reaction from a single copy based on counting fluorescence released microchambers. Anal Chem 76:6434–6439
Poritz MA, Blaschke AJ, Byington CL, Meyers L, Nilsson K, Jones DE, Thatcher SA, Robbins T, Lingenfelter B, Amiott E, Herbener A, Daly J, Dobrowolski SF, Teng DH-F, Ririe KM (2011) FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS ONE 6(10):e26047
Rissin DM et al (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28:595–599
Schuler F, Trotter M, Geltman M, Schwemmer F, Wadle S, Dominguez-Garrido E, Lopez M, Cervera-Acedo C, Santibanez P, von Stetten F, Zengerle R, Paust N (2016) Digital droplet PCR on disk. Lab Chip 16:208–216
Shen F, Du W, Kreutz J, Fok A, Ismagilov R (2010) Digital PCR on a SlipChip. Lab Chip 10:2666–2672
Sposito AJ, DeVoe DL (2017) Staggered trap arrays for robust microfluidic sample digitization. Lab Chip 17:4105–4112
Strohmeier O, Keller M, Schwemmer F, Zehnle S, Mark D, von Stetten F, Zengerle R, Paust N (2015) Centrifugal microfluidic platforms: advanced unit operations and applications. Chem Soc Rev 44:6187–6229
Takulapalli B et al (2012) High density diffusion-free nanowell arrays. J Proteome Res 18:4382–4391
van Doorn R, Szemes M, Bonants P, Kowalchuk G, Salles J, Ortenberg E, Schoen C (2007) Quantitative multiplex detection of plant pathogens using a novel ligation probe-based system coupled with universal, high-throughput real-time PCR on OpenArrays. BMC Genomics 8:276
Vulto P, Podszun S, Meyer P, Hermann C, Manz A, Urban G (2011) Phaseguides: a paradigm shift in microfluidic priming and emptying. Lab Chip 11(9):1596–1602
Wang Y, Sims C, Allbritton N (2012) Dissolution-guided wetting for microarray and microfluidic devices. Lab Chip 12:3036–3039
Wondimu SF, von der Ecken S, Ahrens R, Freude W, Guber AE, Koos C (2017) Integration of digital microfluidics with whispering-gallery mode sensors for label-free detection of biomolecules. Lab Chip 17:1740–1748
Zengerle R, Leitner M, Kluge S, Richter A (1995) Carbon dioxide priming of micro liquid systems. In: Proc. of IEEE-conf. on MEMS ’95, pp 340–343
Zhang H, Nie S, Etson C, Wang R, Walt D (2012) Oil-sealed femtoliter fiber-optic arrays for single molecule analysis. Lab Chip 12:2229–2239
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Podbiel, D., Zengerle, R. & Hoffmann, J. An analytical model for void-free priming of microcavities. Microfluid Nanofluid 24, 16 (2020). https://doi.org/10.1007/s10404-020-2318-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-020-2318-7