Abstract
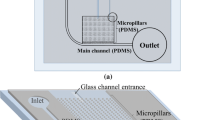
Automated generation of a thin blood smear with a pumpless, capillary-driven, microfluidic would overcome the limitations of manually prepared smears and enable potential point-of-care (POC) applications. Herein, this was accomplished with microfluidic design that leveraged an amphiphilic silicone and channel pillars. The silicone (Sylgard 184) was combined with a surface-modifying additive (SMA), an amphiphilic poly(ethylene oxide) (PEO)-silane at varying concentrations (3, 5, and 7 wt%). The channels were formed with dimensions of 4.7 µm, 250 µm, and 16 mm (height × width × length, respectively). Pillar sections were added at the inlet, outlet and two interior sections to not only prevent channel collapse but to improve flow and cell distribution. After deposition of blood (0.3, 1, and 2 µL) to the channel inlet, the flow time and flow stop times were recorded and the channels imaged to assess smear uniformity and for automated cell counting. A thin blood smear was generated for microfluidic chips prepared with 5 wt% SMA and provided with 0.3 µL of blood.




Similar content being viewed by others
References
Adewoyin AS, Nwogoh B (2014) Peripheral blood film—a review. Ann Ib Postgrad Med 12(2):71–79
Bain BJ (2005) Diagnosis from the blood smear. N Engl J Med 353(5):498–507
Benattar L, Flandrin G (1999) Comparison of the classical manual pushed wedge films, with an improved automated method for making blood smears. Hematol Cell Ther 41(5):211–215
Biadglegne F, Belyhun Y, Ali J, Walle F, Gudeta N, Kassu A, Mulu A (2014) Does the practice of blood film microscopy for detection and quantification of malaria parasites in northwest ethiopia fit the standard? BMC Health Serv Res 14:529
Ceelie H, Dinkelaar RB, van Gelder W (2007) Examination of peripheral blood films using automated microscopy; evaluation of diffmaster octavia and cellavision Dm96. J Clin Pathol 60(1):72–79
Chantiwas R, Park S, Soper SA, Kim BC, Takayama S, Sunkara V, Hwang H, Cho YK (2011) Flexible fabrication and applications of polymer nanochannels and nanoslits. Chem Soc Rev 40(7):3677–3677
Clodfelter RL Jr (1986) The peripheral smear. Emerg Med Clin N Am 4(1):59–74
Comar SR, Malvezzi M, Pasquini R (2017) Evaluation of criteria of manual blood smear review following automated complete blood counts in a large University Hospital. Rev Bras Hematol Hemoter 39(4):306–317
Dogbevi KS, Ngo BKD, Blake CW, Grunlan MA, Coté GL (2020) Pumpless, “Self-Driven” microfluidic channels with controlled blood flow using an amphiphilic silicone. ACS Appl Polym Mater. https://doi.org/10.1021/acsapm.0c00249
Dowling MA, Shute GT (1966) A comparative study of thick and thin blood films in the diagnosis of scanty malaria parasitaemia. B World Health Organ 34(2):249–267
Du X, Zhang P, Liu Y, Wu Y (2011) A passive through hole microvalve for capillary flow control in microfluidic systems. Sens Actuators A Phys 165(2):288–293
Fatona A, Chen Y, Reid M, Brook MA, Moran-Mirabal JM (2015) One-step in-mould modification of pdms surfaces and its application in the fabrication of self-driven microfluidic channels. Lab Chip 15(22):4322–4330
Fuentes-Arderiu X, García-Panyella M, Dot-Bach D (2007) Between-examiner reproducibility in manual differential leukocyte counting. Accredit Qual Assur 12(12):643–645
Green JE, Weintraub HA, Donnelly BS, Mordecai BG (1979) Sample preparation variation and its effects on automated blood cell differential analysis. Anal Quant Cytol 1(3):187–201
Gulati G, Song J, Florea AD, Gong J (2013) Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med 33(1):1–7
Hale RS, Bonnecaze RT, Hidrovo CH (2014a) Optimization of capillary flow through square micropillar arrays. Int J Multiphas Flow 58:39–51
Hale RS, Ranjan R, Hidrovo CH (2014b) Capillary flow through rectangular micropillar arrays. Int J Heat Mass Tran 75:710–717
Horning MP, Delahunt CB, Singh SR, Garing SH, Nichols KP (2014) A paper microfluidic cartridge for automated staining of malaria parasites with an optically transparent microscopy window. Lab Chip 14(12):2040
Houwen B (2002) Blood film preparation and staining procedures. Clin Lab Med 22(1):1–14
Kaminaga M, Ishida T, Kadonosono T, Kizaka-Kondoh S, Omata T (2015) Uniform cell distribution achieved by using cell deformation in a micropillar array. Micromachines (basel) 6(4):409–422
Kaminaga M, Ishida T, Kadonosono T, Kizaka-Kondoh S, Omata T (2019) Microfluidic device for screening for target cell-specific binding molecules by using adherent cells. Micromachines (basel). https://doi.org/10.3390/mi10010041
Kull JA, Krawczel PD, Pighetti GM (2018) Short communication: evaluation of an automated method for assessing white blood cell concentrations in holstein dairy cows. Vet Immunol Immunopathol 197:21–23
Lehmann M, Wallbank AM, Dennis KA, Wufsus AR, Davis KM, Rana K, Neeves KB (2015) On-Chip Recalcification of citrated whole blood using a microfluidic herringbone mixer. Biomicrofluidics 9(6):064106–064106
Li H, Jayamohan H, Lambert C, Mohanty S, Gale BK (2013) Automated whole blood processing with a portable microfluidic device for point-of-care diagnosis (October). pp 1758–1760
Maria MS, Rakesh PE, Chandra TS, Sen AK (2017) Capillary flow-driven microfluidic device with wettability gradient and sedimentation effects for blood plasma separation. Sci Rep UK 7:43457–43457
Marimuthu M, Kim S (2013) Pumpless steady-flow microfluidic chip for cell culture. Anal Biochem 437(2):161–163
Melin J, Quake SR (2007) Microfluidic large-scale integration: the evolution of design rules for biological automation. Annu Rev Biophys Biomol 36:213–231
Mielczarek WS, Obaje EA, Bachmann TT, Kersaudy-Kerhoas M (2016) microfluidic blood plasma separation for medical diagnostics: is it worth it? Lab Chip 16(18):3441–3448
Moen ST, Hatcher CL, Singh AK (2016) A centrifugal microfluidic platform that separates whole blood samples into multiple removable fractions due to several discrete but continuous density gradient sections. PLoS ONE 11(4):e0153137
Nourbakhsh M, Atwood JG, Raccio J, Seligson D (1978) An evaluation of blood smears made by a new method using a spinner and diluted blood. Am J Clin Pathol 70(6):885–892
Organization WH (2016) Malaria microscopy quality assurance manual-version 2. World Health Organization
Park S, Shabani R, Schumacher M, Kim Y-S, Bae YM, Lee K-H, Cho HJ (2016) On-chip whole blood plasma separator based on microfiltration, sedimentation and wetting contrast. Microsyst Technol 22(8):2077–2085
Riedl J (2018) Digital imaging/morphology is the next chapter in hematology. Med Lab Obs 50(3):28–32
Roland L, Drillich M, Iwersen M (2014) Hematology as a diagnostic tool in bovine medicine. J Vet Diagn Invest 26(5):592–598
Rosenthal DS, Schrier S, Timauer J (2002) Evalutaion of the peripheral blood smear. UpToDate
Rufin MA, Ngo BKD, Barry ME, Page VM, Hawkins ML, Stafslien SJ, Grunlan MA (2017) Antifouling silicones based on surface-modifying additive amphiphiles. Green Mater 5(1):1–10
Saha AA, Mitra SK, Tweedie M, Roy S, McLaughlin J (2009) Experimental and Numerical investigation of capillary flow in Su8 and Pdms microchannels with integrated pillars. Microfluid Nanofluid 7(4):451–465
Saurabh V (2006) Manipulating fluids: advances in micro-fluidics, opto-fluidics and fluidic self assembly. Ph.D. Thesis, California Institute of Technology
Simson E, Gascon-Lema MG, Brown DL (2010) Performance of automated slidemakers and stainers in a working laboratory environment—routine operation and quality control. Int J Lab Hematol 32(1 Pt 1):e64–76
Sio SWS, Sun W, Kumar S, Bin WZ, Tan SS, Ong SH, Kikuchi H, Oshima Y, Tan KSW (2007) Malariacount: an image analysis-based program for the accurate determination of parasitemia. J Microbiol Meth 68(1):11–18
Smith S, Madzivhandila P, Sewart R, Govender U, Becker H, Roux P, Land K (2017) Microfluidic cartridges for automated, point-of-care blood cell counting. SLAS Technol 22(2):176–185
Suk JW, Cho J-H (2007) Capillary flow control using hydrophobic patterns. J Micromech Microeng 17(4):N11–N15
Thangawng AL, Swartz MA, Glucksberg MR, Ruoff RS (2007) Bond-detach lithography: a method for micro/nanolithography by precision PDMS patterning. Small 3(1):132–138
Thorslund S, Larsson R, Bergquist J, Nikolajeff F, Sanchez J (2008) A Pdms-based disposable microfluidic sensor for Cd4+ lymphocyte counting. Biomed Microdevices 10(6):851–857
Tripathi S, Varun Kumar YVB, Prabhakar A, Joshi SS, Agrawal A (2015) Passive blood plasma separation at the microscale: a review of design principles and microdevices. J Micromech Microeng 25(8):083001–083001
Wang D, Chan HN, Liu Z, Micheal S, Li L, Baniani DB, Tan MJ, Huang L, Wang J, Wu H (2020) Recent developments in microfluidic‐based point‐of‐care testing (Poct) diagnoses. Microfluid Nanofluidics 239–280
World Health Organization, Regional Office for the Western (2016) Malaria microscopy standard operating procedures. WHO Regional Office for the Western Pacific, Manila
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH (2007) A review of malaria diagnostic tools: microscopy and rapid diagnostic test (Rdt). Am J Trop Med Hyg 77(6_Suppl):119–127
Yao M, Fang J (2012) Hydrophilic peo-Pdms for microfluidic applications. J Micromech Microeng 22(2):25012
Ye Z, Li S, Wang C, Shen R, Wen W (2016) Capillary flow control in nanochannels via hybrid surface. RSC Adv 6(4):2774–2777
Acknowledgements
G.L.C. gratefully acknowledges support from the National Science Foundation (#1402846). K.S.D. gratefully acknowledges support from the National Science Foundation (#HRD-1502335). All authors acknowledge support from the Texas A&M Engineering Experiment Station (TEES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dogbevi, K.S., Ngo, B.K.D., Branan, K.L. et al. A thin whole blood smear prepared via pumpless microfluidics. Microfluid Nanofluid 25, 59 (2021). https://doi.org/10.1007/s10404-021-02457-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-021-02457-4