Abstract
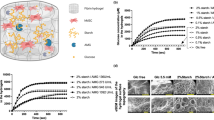
Adult hematopoietic stem cells (HSCs) produce the body’s full complement of blood and immune cells. They reside in specialized microenvironments, or niches, within the bone marrow. The perivascular niche near blood vessels is believed to help maintain primitive HSCs in an undifferentiated state but demonstration of this effect is difficult. In vivo studies make it challenging to determine the direct effect of the endosteal and perivascular niches as they can be in close proximity, and two-dimensional in vitro cultures often lack an instructive extracellular matrix environment. We describe a tissue engineering approach to develop and characterize a three-dimensional perivascular tissue model to investigate the influence of the perivascular secretome on HSC behavior. We generate 3D endothelial networks in methacrylamide-functionalized gelatin hydrogels using human umbilical vein endothelial cells (HUVECs) and mesenchymal stromal cells (MSCs). We identify a subset of secreted factors important for HSC function, and examine the response of primary murine HSCs in hydrogels to the perivascular secretome. Within 4 days of culture, perivascular conditioned media promoted maintenance of a greater fraction of hematopoietic stem and progenitor cells. This work represents an important first-generation perivascular model to investigate the role of niche secreted factors on the maintenance of primary HSCs.




Similar content being viewed by others
References
Acar, M., K. S. Kocherlakota, M. M. Murphy, J. G. Peyer, H. Oguro, C. N. Inra, C. Jaiyeola, Z. Zhao, K. Luby-Phelps, and S. J. Morrison. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526:126–130, 2015.
Asada, N., Y. Kunisaki, H. Pierce, Z. Wang, N. F. Fernandez, A. Birbrair, A. Maayan, and P. S. Frenette. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 19:214–223, 2017.
Bartling, B., A. Koch, A. S. Robert, S. R.-E. Silber, and A. N. Santos. Insulin-like growth factor binding proteins-2 and -4 enhance the migration of human CD34−/CD133+ hematopoietic stem and progenitor cells. Int. J. Mol. Med. 25:89–96, 2010.
Boussommier-Calleja, A., Y. Atiyas, K. Haase, M. Headley, C. Lewis, and R. D. Kamm. The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials 198:180–193, 2019.
Braham, M. V. J., A. S. P. Li Yim, J. Garcia Mateos, M. C. Minnema, W. J. A. Dhert, F. C. Öner, C. Robin, and J. Alblas. A human hematopoietic niche model supporting hematopoietic stem and progenitor cells in vitro. Adv. Healthcare Mater. 8:1801444, 2019.
Bryder, D., D. J. Rossi, and I. L. Weissman. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 169:338–346, 2006.
Burke, M. D., J. O. Park, M. Srinivasarao, and S. A. Khan. A novel enzymatic technique for limiting drug mobility in a hydrogel matrix. J. Controlled Rel. 104:141–153, 2005.
Butler, J. M., D. J. Nolan, E. L. Vertes, B. Varnum-Finney, H. Kobayashi, A. T. Hooper, M. Seandel, K. Shido, I. A. White, M. Kobayashi, L. Witte, C. May, C. Shawber, Y. Kimura, J. Kitajewski, Z. Rosenwaks, I. D. Bernstein, and S. Rafii. Endothelial cells are essential for the self-renewal and repopulation of notch-dependent hematopoietic stem cells. Cell Stem Cell 6:251–264, 2010.
Campisi, M., Y. Shin, T. Osaki, C. Hajal, V. Chiono, and R. D. Kamm. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180:117–129, 2018.
Carlson, P., A. Dasgupta, C. A. Grzelak, J. Kim, A. Barrett, I. M. Coleman, R. E. Shor, E. T. Goddard, J. Dai, E. M. Schweitzer, A. R. Lim, S. B. Crist, D. A. Cheresh, P. S. Nelson, K. C. Hansen, and C. M. Ghajar. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21:238–250, 2019.
Çelebi, B., D. Mantovani, and N. Pineault. Insulin-like growth factor binding protein-2 and neurotrophin 3 synergize together to promote the expansion of hematopoietic cells ex vivo. Cytokine 58:327–331, 2012.
Challen, G. A., N. Boles, K. K. Lin, and M. A. Goodell. Mouse hematopoietic stem cell identification and analysis. Cytom Part A 75:14–24, 2009.
Cheuk, D. K. Optimal stem cell source for allogeneic stem cell transplantation for hematological malignancies. World J. Transplant. 3:99–112, 2013.
Chou, S., and H. F. Lodish. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. PNAS 107:7799–7804, 2010.
Christodoulou, C., J. A. Spencer, S.-C. A. Yeh, R. Turcotte, K. D. Kokkaliaris, R. Panero, A. Ramos, G. Guo, N. Seyedhassantehrani, T. V. Esipova, S. A. Vinogradov, S. Rudzinskas, Y. Zhang, A. S. Perkins, S. H. Orkin, R. A. Calogero, T. Schroeder, C. P. Lin, and F. D. Camargo. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature 578:278–283, 2020.
Crosby, C., D. Valliappan, S. K. David Shu, W. D. Chengyi Tu, S. H. Parekh, and J. Zoldan. Quantifying the vasculogenic potential of induced pluripotent stem cell-derived endothelial progenitors in collagen hydrogels. Tissue Eng. Part A 25:746–758, 2019.
Crosby, C. O., and J. Zoldan. An in vitro 3D model and computational pipeline to quantify the vasculogenic potential of iPSC-derived endothelial progenitors. J. Vis. Exp. 2019. https://doi.org/10.3791/59342.
Danby, R., and V. Rocha. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front. Immunol. 5:68, 2014.
Derakhshani, M., H. Abbaszadeh, A. A. Movassaghpour, A. Mehdizadeh, M. Ebrahimi-Warkiani, and M. Yousefi. Strategies for elevating hematopoietic stem cells expansion and engraftment capacity. Life Sci. 232:116598, 2019.
Ding, L., T. L. Saunders, G. Enikolopov, and S. J. Morrison. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481:457–462, 2012.
D’Souza, A., S. Lee, X. Zhu, and M. Pasquini. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 23:1417–1421, 2017.
Easteal, A. J., W. E. Price, and L. A. Woolf. Diaphragm cell for high-temperature diffusion measurements Tracer Diffusion coefficients for water to 363 K. J. Chem. Soc. Faraday Trans. 1(85):1091–1097, 1989.
Fleming, H. E., V. Janzen, C. LoCelso, J. Guo, K. M. Leahy, H. M. Kronenberg, and D. T. Scadden. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2:274–283, 2008.
Ghajar, C. M., H. Peinado, H. Mori, I. R. Matei, K. J. Evason, H. Brazier, D. Almeida, A. Koller, K. A. Hajjar, D. Y. R. Stainier, E. I. Chen, D. Lyden, and M. J. Bissell. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15:807, 2013.
Gilchrist A. E., Harley B. Connecting secretome to hematopoietic stem cell phenotype shifts in an engineered bone marrow niche. Integr Biol (Camb). 12(7):175–187, 2020.
Gilchrist, A. E., S. Lee, Y. Hu, and B. A. C. Harley. Soluble signals and remodeling in a synthetic gelatin-based hematopoietic stem cell niche. Adv. Healthcare Mater. 8:1900751, 2019.
Gomei, Y., Y. Nakamura, H. Yoshihara, K. Hosokawa, H. Iwasaki, T. Suda, and F. Arai. Functional differences between two Tie2 ligands, angiopoietin-1 and -2, in regulation of adult bone marrow hematopoietic stem cells. Exp. Hematol. 38:82–89.e1, 2010.
Goncalves, K. A., L. Silberstein, S. Li, N. Severe, M. G. Hu, H. Yang, D. T. Scadden, and G.-F. Hu. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell 166:894–906, 2016.
Haase, K., M. R. Gillrie, C. Hajal, and R. D. Kamm. Pericytes contribute to dysfunction in a human 3D model of placental microvasculature through VEGF-Ang-Tie2 signaling. Adv. Sci. (Weinh) 6:1900878, 2019.
Hagman, J., N. Lorén, and A.-M. Hermansson. Effect of gelatin gelation kinetics on probe diffusion determined by FRAP and rheology. Biomacromolecules 11:3359–3366, 2010.
Himburg, H. A., P. L. Doan, M. Quarmyne, X. Yan, J. Sasine, L. Zhao, G. V. Hancock, J. Kan, K. A. Pohl, E. Tran, N. J. Chao, J. R. Harris, and J. P. Chute. Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nat. Med. 23:91, 2016.
Huynh, H., J. Zheng, M. Umikawa, C. Zhang, R. Silvany, S. Iizuka, M. Holzenberger, W. Zhang, and C. C. Zhang. IGF binding protein 2 supports the survival and cycling of hematopoietic stem cells. Blood 118:3236–3243, 2011.
Ibrahim, A. A., T. Yahata, M. Onizuka, T. Dan, C. van Ypersele De Strihou, T. Miyata, and K. Ando. Inhibition of plasminogen activator inhibitor type-1 activity enhances rapid and sustainable hematopoietic regeneration. Stem Cells 32:946–958, 2014.
Itkin, T., S. Gur-Cohen, J. A. Spencer, A. Schajnovitz, S. K. Ramasamy, A. P. Kusumbe, G. Ledergor, Y. Jung, I. Milo, M. G. Poulos, A. Kalinkovich, A. Ludin, O. Kollet, G. Shakhar, J. M. Butler, S. Rafii, R. H. Adams, D. T. Scadden, C. P. Lin, and T. Lapidot. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532:323–328, 2016.
Janes, K., J. Kelly, S. Gaudet, J. Albeck, P. Sorger, and D. Lauffenburger. Cue-signal-response analysis of TNF-induced apoptosis by partial least squares regression of dynamic multivariate data. J. Comput. Biol. 11:544–561, 2004.
Jansen, L. E., N. P. Birch, J. D. Schiffman, A. J. Crosby, and S. R. Peyton. Mechanics of intact bone marrow. J. Mech. Behav. Biomed. Mater. 50:299–307, 2015.
Jeon, J. S., S. Bersini, M. Gilardi, G. Dubini, J. L. Charest, M. Moretti, and R. D. Kamm. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl. Acad. Sci. USA 112:214–219, 2015.
Jönsson, P., M. P. Jonsson, J. O. Tegenfeldt, and F. Höök. A method improving the accuracy of fluorescence recovery after photobleaching analysis. Biophys. J. 95:5334–5348, 2008.
Kirito, K., N. Fox, and K. Kaushansky. Thrombopoietin stimulates Hoxb4 expression: an explanation for the favorable effects of TPO on hematopoietic stem cells. Blood 102:3172–3178, 2003.
Kobayashi, H., J. M. Butler, R. O’Donnell, M. Kobayashi, B. S. Ding, B. Bonner, V. K. Chiu, D. J. Nolan, K. Shido, L. Benjamin, and S. Rafii. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12:1046–1056, 2010.
Kreeger, P. K. Using partial least squares regression to analyze cellular response data. Sci. Signal 6:tr7-tr7, 2013.
Kunisaki, Y., I. Bruns, C. Scheiermann, J. Ahmed, S. Pinho, D. Zhang, T. Mizoguchi, Q. Wei, D. Lucas, K. Ito, J. C. Mar, A. Bergman, and P. S. Frenette. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502:637, 2013.
Mahadik, B. P., N. A. K. Bharadwaj, R. H. Ewoldt, and B. A. C. Harley. Regulating dynamic signaling between hematopoietic stem cells and niche cells via a hydrogel matrix. Biomaterials 125:54–64, 2017.
Mahadik, B. P., S. PedronHaba, L. J. Skertich, and B. A. C. Harley. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials 67:297–307, 2015.
Malhotra, S., and P. W. Kincade. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp. Hematol. 37:19–30, 2009.
McGill, M., J. M. Coburn, B. P. Partlow, X. Mu, and D. L. Kaplan. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design. Acta Biomater. 63:76–84, 2017.
Mendez-Ferrer, S., T. V. Michurina, F. Ferraro, A. R. Mazloom, B. D. Macarthur, S. A. Lira, D. T. Scadden, A. Ma’ayan, G. N. Enikolopov, and P. S. Frenette. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834, 2010.
Ngo, M. T., and B. A. Harley. The influence of hyaluronic acid and glioblastoma cell coculture on the formation of endothelial cell networks in gelatin hydrogels. Adv. Healthcare Mater. 6:1700687, 2017.
Ngo, M. T., and B. A. C. Harley. The influence of hyaluronic acid and glioblastoma cell co-culture on the formation of endothelial cell networks in gelatin hydrogels. Adv. Healthcare Mater. 6:1700687, 2017.
Ngo, M. T., and B. A. C. Harley. Perivascular signals alter global gene expression profile of glioblastoma and response to temozolomide in a gelatin hydrogel. Biomaterials 198:122–134, 2019.
Ngo, M. T., E. Karvelis, and B. A. C. Harley. Multidimensional hydrogel models reveal endothelial network angiocrine signals increase glioblastoma cell number, invasion, and temozolomide resistance. Integr. Biol. (Camb) 12:139–149, 2020.
Nilsson, S. K., H. M. Johnston, G. A. Whitty, B. Williams, R. J. Webb, D. T. Denhardt, I. Bertoncello, L. J. Bendall, P. J. Simmons, and D. N. Haylock. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106:1232–1239, 2005.
Nombela-Arrieta, C., G. Pivarnik, B. Winkel, K. J. Canty, B. Harley, J. E. Mahoney, S.-Y. Park, J. Lu, A. Protopopov, and L. E. Silberstein. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat. Cell Biol. 15:533, 2013.
Offeddu, G. S., Y. Shin, and R. D. Kamm. Microphysiological models of neurological disorders for drug development. Curr. Opin. Biomed. Eng. 13:119–126, 2020.
Osaki, T., S. G. M. Uzel, and R. D. Kamm. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 1:1, 2018.
Ozdemir, Z. N., and Bozdağ S. Civriz. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus. Apher. Sci. 57:163–167, 2018.
Pedron, S., E. Becka, and B. A. C. Harley. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials 34:7408–7417, 2013.
Pedron, S., and B. A. C. Harley. Impact of the biophysical features of a 3D gelatin microenvironment on glioblastoma malignancy. J. Biomed. Mater. Res. Part A 101:3404–3415, 2013.
Pinho, S., and P. S. Frenette. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20:303–320, 2019.
Poulos, M. G., M. J. P. Crowley, M. C. Gutkin, P. Ramalingam, W. Schachterle, J. L. Thomas, O. Elemento, and J. M. Butler. Vascular platform to define hematopoietic stem cell factors and enhance regenerative hematopoiesis. Stem Cell Reports 5:881–894, 2015.
Poulos Michael, G., P. Guo, M. Kofler Natalie, S. Pinho, C. Gutkin Michael, A. Tikhonova, I. Aifantis, S. Frenette Paul, J. Kitajewski, S. Rafii, and M. Butler Jason. Endothelial jagged-1 Is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 4:1022–1034, 2013.
Robinson, S. N., J. Ng, T. Niu, H. Yang, J. D. McMannis, S. Karandish, I. Kaur, P. Fu, M. Del Angel, R. Messinger, F. Flagge, M. de Lima, W. Decker, D. Xing, R. Champlin, and E. J. Shpall. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 37:359–366, 2006.
Shirahama, H., B. H. Lee, L. P. Tan, and N.-J. Cho. Precise tuning of facile one-pot gelatin methacryloyl (GelMA) synthesis. Sci. Rep. 6:31036, 2016.
Silberstein, L., K. A. Goncalves, P. V. Kharchenko, R. Turcotte, Y. Kfoury, F. Mercier, N. Baryawno, N. Severe, J. Bachand, J. A. Spencer, A. Papazian, D. Lee, B. R. Chitteti, E. F. Srour, J. Hoggatt, T. Tate, C. LoCelso, N. Ono, S. Nutt, J. Heino, K. Sipila, T. Shioda, M. Osawa, C. P. Lin, G. F. Hu, and D. T. Scadden. Proximity-based differential single-cell analysis of the niche to identify stem/progenitor cell regulators. Cell Stem Cell 19:530–543, 2016.
Silberstein, L. E., and C. P. Lin. A new image of the hematopoietic stem cell vascular niche. Cell Stem Cell 13:514–516, 2013.
Spill, F., D. S. Reynolds, R. D. Kamm, and M. H. Zaman. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 40:41–48, 2016.
Szklarczyk, D., A. L. Gable, D. Lyon, A. Junge, S. Wyder, J. Huerta-Cepas, M. Simonovic, N. T. Doncheva, J. H. Morris, P. Bork, L. J. Jensen, and V. Mering Christian. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47:607–613, 2018.
Tian, C., and Y. Zhang. Purification of hematopoietic stem cells from bone marrow. Ann. Hematol. 95:543–547, 2016.
Tsirkinidis, P., E. Terpos, G. Boutsikas, A. Papatheodorou, K. Anargyrou, E. Lalou, A. Dimitrakopoulou, C. Kalpadakis, K. Konstantopoulos, M. Siakantaris, P. Panayiotidis, G. Pangalis, M.-C. Kyrtsonis, T. Vassilakopoulos, and M. K. Angelopoulou. Bone metabolism markers and angiogenic cytokines as regulators of human hematopoietic stem cell mobilization. J. Bone Min. Metab. 36:399–409, 2018.
Wold, S., M. Sjostrom, and L. Eriksson. PLS-regression: a basic tool of chemometrics. Chemom. Intell Lab. Syst. 58:109–130, 2001.
Xu, C., X. Gao, Q. Wei, F. Nakahara, S. E. Zimmerman, J. Mar, and P. S. Frenette. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat. Commun. 9:2449, 2018.
Yahata, T., A. A. Ibrahim, Y. Muguruma, M. Eren, A. M. Shaffer, N. Watanabe, S. Kaneko, T. Nakabayashi, T. Dan, N. Hirayama, D. E. Vaughan, T. Miyata, and K. Ando. TGF-β–induced intracellular PAI-1 is responsible for retaining hematopoietic stem cells in the niche. Blood 130:2283–2294, 2017.
Yang, L., D. Bryder, J. R. Adolfsson, J. Nygren, R. Månsson, M. Sigvardsson, and S. E. W. Jacobsen. Identification of Lin–Sca1+kit+CD34+Flt3– short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105:2717–2723, 2005.
Zhang, C. C., M. Kaba, G. Ge, K. Xie, W. Tong, C. Hug, and H. F. Lodish. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 12:240–245, 2006.
Acknowledgments
The authors would like to acknowledge Dr. Barbara Pilas of the Roy J. Carver Biotechnology Center (Flow Cytometry Facility, UIUC) for assistance with bone marrow cell isolation and flow cytometry. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers R01 DK099528 (B.A.C.H), as well as by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers R21 EB018481 (B.A.C.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors are also grateful for additional funding provided by the Department of Chemical & Biomolecular Engineering and the Institute for Genomic Biology at the University of Illinois at Urbana-Champaign. The authors would like to acknowledge Zona Hrnjak and Aidan Gilchrist for the development of a custom Matlab code for rapid analysis of mechanical testing data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer West oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barnhouse, V., Petrikas, N., Crosby, C. et al. Perivascular Secretome Influences Hematopoietic Stem Cell Maintenance in a Gelatin Hydrogel. Ann Biomed Eng 49, 780–792 (2021). https://doi.org/10.1007/s10439-020-02602-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02602-0