Abstract
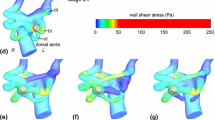
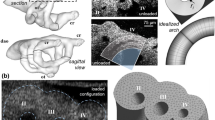
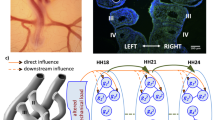
Mechanical forces are essential for proper growth and remodeling of the primitive pharyngeal arch arteries (PAAs) into the great vessels of the heart. Despite general acknowledgement of a hemodynamic-malformation link, the direct correlation between hemodynamics and PAA morphogenesis remains poorly understood. The elusiveness is largely due to difficulty in performing isolated hemodynamic perturbations and quantifying changes in-vivo. Previous in-vivo arch artery occlusion/ablation experiments either did not isolate the effects of hemodynamics, did not analyze the results in a 3D context or did not consider the effects of varying degrees of occlusion. Here, we overcome these limitations by combining minimally invasive occlusion experiments in the avian embryo with 3D anatomical models of development and in-silico testing of experimental phenomenon. We detail morphological and hemodynamic changes 24 hours post vessel occlusion. 3D anatomical models showed that occlusion geometries had more circular cross-sectional areas and more elongated arches than their control counterparts. Computational fluid dynamics revealed a marked change in wall shear stress-morphology trends. Instantaneous (in-silico) occlusion models provided mechanistic insights into the dynamic vessel adaptation process, predicting pressure-area trends for a number of experimental occlusion arches. We follow the propagation of small defects in a single embryo Hamburger Hamilton (HH) Stage 18 embryo to a more serious defect in an HH29 embryo. Results demonstrate that hemodynamic perturbation of the presumptive aortic arch, through varying degrees of vessel occlusion, overrides natural growth mechanisms and prevents it from becoming the dominant arch of the aorta.








Similar content being viewed by others
References
Bajolle, F., S. Zaffran, R. G. Kelly, J. Hadchouel, D. Bonnet, N. A. Brown, and M. E. Buckingham. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ. Res. 98:421–428, 2006.
Bockman, D. E., M. E. Redmond, K. Waldo, H. Davis, and M. L. Kirby. Effect of neural crest ablation on development of the heart and arch arteries in the chick. Am. J. Anat. 180:332–341, 1987.
De Clercq, K., E. Persoons, T. Napso, C. Luyten, T. N. Parac-Vogt, A. N. Sferruzzi-Perri, G. Kerckhofs, and J. Vriens. High-resolution contrast-enhanced microCT reveals the true three-dimensional morphology of the murine placenta. Proc. Natl. Acad. Sci. U. S. A. 116:13927–13936, 2019.
deAlmeida, A., T. McQuinn, and D. Sedmera. Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ. Res. 100:1363–1370, 2007.
Fisher, A. B., S. Chien, A. I. Barakat, and R. M. Nerem. Endothelial cellular response to altered shear stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L529–L533, 2001.
Girerd, X., G. London, P. Boutouyrie, J.-J. Mourad, M. Safar, and S. Laurent. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension 27:799–803, 1996.
Go, A. S., et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127:e6–e245, 2013.
Harh, J. Y., M. H. Paul, W. J. Gallen, D. Z. Friedberg, and S. Kaplan. Experimental production of hypoplastic left heart syndrome in the chick embryo. Am. J. Cardiol. 31:51–56, 1973.
Hiruma, T., and R. Hirakow. Formation of the pharyngeal arch arteries in the chick embryo. Observations of corrosion casts by scanning electron microscopy. Anat. Embryol. 191:415–423, 1995.
Hiruma, T., Y. Nakajima, and H. Nakamura. Development of pharyngeal arch arteries in early mouse embryo. J. Anat. 201:15–29, 2002.
Hogers, B., M. C. C. DeRuiter, A. C. C. Gittenberger-de Groot, and R. E. E. Poelmann. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ. Res. 80:473–481, 1997.
Hogers, B., M. C. DeRuiter, A. C. Gittenberger-de Groot, and R. E. Poelmann. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc. Res. 41:87–99, 1999.
Hove, J. R., R. W. Köster, A. S. Forouhar, G. Acevedo-Bolton, S. E. Fraser, and M. Gharib. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421:172–177, 2003.
Hu, N., D. A. Christensen, A. K. Agrawal, C. Beaumont, E. B. Clark, and J. A. Hawkins. Dependence of aortic arch morphogenesis on intracardiac blood flow in the left atrial ligated chick embryo. Anat. Rec. 292:652–660, 2009.
Hu, N., and E. Clark. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ. Res. 65:1665–1670, 1989.
Kamiya, A., and T. Togawa. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 8:14–21, 1980.
Kowalski, W. J., O. Dur, Y. Wang, M. J. Patrick, J. P. Tinney, B. B. Keller, and K. Pekkan. Critical transitions in early embryonic aortic arch patterning and hemodynamics. PLoS ONE 8:2013.
Kuratani, S. C., and M. L. Kirby. Initial migration and distribution of the cardiac neural crest in the avian embryo: an introduction to the concept of the circumpharyngeal crest. Am. J. Anat. 191:215–227, 1991.
Lindsey, S. E., P. G. Menon, W. J. Kowalski, A. Shekhar, H. C. Yalcin, N. Nishimura, C. B. Schaffer, J. T. Butcher, and K. Pekkan. Growth and hemodynamics after early embryonic aortic arch occlusion. Biomech. Model. Mechanobiol. 14:735–751, 2015.
Lindsey, S. E., J. T. Butcher, and I. E. Vignon-Clementel. Cohort-based multiscale analysis of hemodynamic-driven growth and remodeling of the embryonic pharyngeal arch arteries. Development 145, 2018.
Liu, C., W. Liu, M. F. Lu, N. A. Brown, and J. F. Martin. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development 128:2039–2048, 2001.
Liu, C., W. Liu, J. Palie, M. F. Lu, N. A. Brown, and J. F. Martin. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development 129:5081–5091, 2002.
Lomonico, M. P., G. W. Moore, and G. M. Hutchins. Rotation of the junction of the outflow tract and great arteries in the embryonic human heart. Anat. Rec. 216:544–549, 1986.
Pant, S., B. Fabrèges, J. F. Gerbeau, and I. E. Vignon-Clementel. A multiscale filtering-based parameter estimation method for patient-specific coarctation simulations in rest and exercise. Lect. Notes Comput. Sci. 8330 LNCS:102–109, 2014.
Pries, A. R., T. W. Secomb, and P. Gaehtgens. Structural adaptation and stability of microvascular networks: theory and simulations. Am. J. Physiol. Hear. Circ. Physiol. 275:349–360, 1998.
Rychter, Z. Experimental morphology of the aortic arches and the heart loop in chick embryos. Adv. Morphog. 1962.
Scherptong, R. W. C., M. R. M. Jongbloed, L. J. Wisse, R. Vicente-Steijn, M. M. Bartelings, R. E. Poelmann, M. J. Schalij, and A. C. Gittenberger-de Groot. Morphogenesis of outflow tract rotation during cardiac development: the pulmonary push concept. Dev. Dyn. 241:1413–1422, 2012.
Sedmera, D., T. Pexieder, V. Rychterova, N. Hu, and E. B. Clark. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat. Rec. 254:238–252, 1999.
Tobita, K., and B. B. Keller. Right and left ventricular wall deformation patterns in normal and left heart hypoplasia chick embryos. Am. J. Physiol. Heart Circ. Physiol. 279:H959–H969, 2000.
Valentin, A., L. Cardamone, S. Baek, and J. D. Humphrey. Mechanical stress exerts control over cellular behavior in the embryo Biomechanical forces connect genetic and molecular-level events to tissue-level deformations. J. R. Soc. Interface 6:293–306, 2009.
Van der Heiden, K., B. C. W. Groenendijk, B. P. Hierck, B. Hogers, H. K. Koerten, A. M. Mommaas, A. C. Gittenberger-de Groot, and R. E. Poelmann. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev. Dyn. 235:19–28, 2006.
Waldo, K. L., M. R. Hutson, C. C. Ward, M. Zdanowicz, H. A. Stadt, D. Kumiski, R. Abu-Issa, and M. L. Kirby. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev. Biol. 281:78–90, 2005.
Waldo, K. L., D. Kumiski, and M. L. Kirby. Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev. Dyn. 205:281–292, 1996.
Waldo, K., S. Miyagawa-Tomita, D. Kumiski, and M. L. Kirby. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev. Biol. 196:129–144, 1998.
Wang, Y., O. Dur, M. J. Patrick, J. P. Tinney, K. Tobita, B. B. Keller, and K. Pekkan. Aortic arch morphogenesis and flow modeling in the chick embryo. Ann. Biomed. Eng. 37:1069–1081, 2009.
Wyczalkowski, M. A., Z. Chen, B. A. Filas, V. D. Varner, and L. A. Taber. Computational models for mechanics of morphogenesis. Birth Defects Res. C. Embryo Today 96:132–152, 2012.
Yalcin, H. C., A. Shekhar, T. C. McQuinn, and J. T. Butcher. Hemodynamic patterning of the avian atrioventricular valve. Dev. Dyn. 240:23–35, 2011.
Yashiro, K., H. Shiratori, and H. Hamada. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450:285–288, 2007.
Yoshigi, M., G. D. Knott, and B. B. Keller. Lumped parameter estimation for the embryonic chick vascular system: a time-domain approach using MLAB. Comput. Methods Programs Biomed. 63:29–41, 2000.
Zarins, C. K., M. A. Zatina, D. P. Giddens, D. N. Ku, and S. Glagov. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J. Vasc. Surg. 5:413–420, 1987.
Acknowledgments
We thank the Cornell BRC imaging facility and Randolph Linderman (reconstructions). This work was funded by NSF GRFP, NSF GROW, an Alfred P. Sloan Foundation fellowship, CMMI-1635712, an Inria International Internship grant and by the National Institutes of Health (HL110328, S10OD012287 and S10OD016191).
Disclosures
The authors have no disclosures.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Electronic supplementary material 2 (AVI 5840 kb)
Electronic supplementary material 3 (AVI 2605 kb)
Rights and permissions
About this article
Cite this article
Lindsey, S.E., Vignon-Clementel, I.E. & Butcher, J.T. Assessing Early Cardiac Outflow Tract Adaptive Responses Through Combined Experimental-Computational Manipulations. Ann Biomed Eng 49, 3227–3242 (2021). https://doi.org/10.1007/s10439-021-02802-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02802-2