Abstract
Lymphangiogenesis is an essential physiological process but also a determining factor in vascular-related pathological conditions. Angiopoietin-2 (Ang2) plays an important role in lymphatic vascular development and function and its upregulation has been reported in several vascular-related diseases, including cancer. Given the established role of the small GTPase RhoA on cytoskeleton-dependent endothelial functions, we investigated the relationship between RhoA and Ang2-induced cellular activities. This study shows that Ang2-driven human dermal lymphatic endothelial cell migration depends on RhoA. We demonstrate that Ang2-induced migration is independent of the Tie receptors, but dependent on β1 integrin-mediated RhoA activation with knockdown, pharmacological approaches, and protein sequencing experiments. Although the key proteins downstream of RhoA, Rho kinase (ROCK) and myosin light chain, were activated, blockade of ROCK did not abrogate the Ang2-driven migratory effect. However, formins, an alternative target of RhoA, were identified as key players, and especially FHOD1. The Ang2-RhoA relationship was explored in vivo, where lymphatic endothelial RhoA deficiency blocked Ang2-induced lymphangiogenesis, highlighting RhoA as an important target for anti-lymphangiogenic treatments.









Similar content being viewed by others
References
Sato TN et al (1993) Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci USA 90(20):9355–9358
Maisonpierre PC et al (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277(5322):55–60
Akwii RG et al (2019) Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells 8(5):471
Tsigkos S, Koutsilieris M, Papapetropoulos A (2003) Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs 12(6):933–941
Davis S et al (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87(7):1161–1169
Hansen TM et al (2010) Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell Signal 22(3):527–532
Teichert-Kuliszewska K et al (2001) Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res 49(3):659–670
Yuan HT et al (2009) Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol 29(8):2011–2022
Kim I et al (2000) Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Oncogene 19(39):4549–4552
Kim M et al (2016) Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J Clin Invest 126(9):3511–3525
Witzenbichler B et al (1998) Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 273(29):18514–18521
Mochizuki Y et al (2002) Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci 115(Pt 1):175–183
Nguyen VP et al (2007) Differential response of lymphatic, venous and arterial endothelial cells to angiopoietin-1 and angiopoietin-2. BMC Cell Biol 8:10
Song SH et al (2012) Tie1 regulates the Tie2 agonistic role of angiopoietin-2 in human lymphatic endothelial cells. Biochem Biophys Res Commun 419(2):281–286
Dumont DJ et al (1995) Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn 203(1):80–92
Partanen J, Dumont DJ (1999) Functions of Tie1 and Tie2 receptor tyrosine kinases in vascular development. Curr Top Microbiol Immunol 237:159–172
Yamaguchi TP et al (1993) flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118(2):489–498
Millauer B et al (1993) High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72(6):835–846
Kaipainen A et al (1993) The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med 178(6):2077–2088
Dumont DJ et al (1992) tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 7(8):1471–1480
Dumont DJ et al (1993) The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene 8(5):1293–1301
Dumont DJ et al (1994) Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8(16):1897–1909
Morisada T et al (2005) Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 105(12):4649–4656
Tammela T et al (2005) Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 105(12):4642–4648
Srinivasan RS et al (2007) Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21(19):2422–2432
Pichol-Thievend C et al (2018) A blood capillary plexus-derived population of progenitor cells contributes to genesis of the dermal lymphatic vasculature during embryonic development. Development. https://doi.org/10.1242/dev.160184
Mofarrahi M, Hussain SN (2011) Expression and functional roles of angiopoietin-2 in skeletal muscles. PLoS One 6(7):e22882
Koga K et al (2001) Expression of angiopoietin-2 in human glioma cells and its role for angiogenesis. Cancer Res 61(16):6248–6254
Stratmann A, Risau W, Plate KH (1998) Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol 153(5):1459–1466
Huang YQ et al (2002) Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood 99(5):1646–1650
Gale NW et al (2002) Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 3(3):411–423
Zhang L et al (2003) Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 63(12):3403–3412
Hackett SF et al (2000) Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol 184(3):275–284
Fiedler U et al (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel–Palade bodies. Blood 103(11):4150–4156
Daly C et al (2006) Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA 103(42):15491–15496
Felcht M et al (2012) Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 122(6):1991–2005
Helaine C et al (2020) Angiopoietin-2 combined with radiochemotherapy impedes glioblastoma recurrence by acting in an autocrine and paracrine manner: a preclinical study. Cancers (Basel) 12(12):3585
Lemieux C et al (2005) Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 105(4):1523–1530
Sfiligoi C et al (2003) Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer 103(4):466–474
Kelly BD et al (2003) Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93(11):1074–1081
Ridley AJ (2015) Rho GTPase signalling in cell migration. Curr Opin Cell Biol 36:103–112
Mikelis CM et al (2013) PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem 288(17):12232–12243
Mikelis CM et al (2015) RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun 6:6725
van Nieuw Amerongen GP et al (2003) Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 23(2):211–217
Zeng H, Zhao D, Mukhopadhyay D (2002) KDR stimulates endothelial cell migration through heterotrimeric G protein Gq/11-mediated activation of a small GTPase RhoA. J Biol Chem 277(48):46791–46798
Bryan BA et al (2010) RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. Faseb J 24(9):3186–3195
Zahra FT et al (2019) Endothelial RhoA GTPase is essential for in vitro endothelial functions but dispensable for physiological in vivo angiogenesis. Sci Rep 9(1):11666
Sainz-Jaspeado M, Claesson-Welsh L (2018) Cytokines regulating lymphangiogenesis. Curr Opin Immunol 53:58–63
Zheng W, Aspelund A, Alitalo K (2014) Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124(3):878–887
Coso S, Bovay E, Petrova TV (2014) Pressing the right buttons: signaling in lymphangiogenesis. Blood 123(17):2614–2624
Kumar B et al (2011) VEGF-C differentially regulates VEGF-A expression in ocular and cancer cells; promotes angiogenesis via RhoA mediated pathway. Angiogenesis 14(3):371–380
He M et al (2010) Vascular endothelial growth factor C promotes cervical cancer metastasis via up-regulation and activation of RhoA/ROCK-2/moesin cascade. BMC Cancer 10:170
Ganguly A et al (2012) Isolation of human umbilical vein endothelial cells and their use in the study of neutrophil transmigration under flow conditions. J Vis Exp 66:e4032
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3(6):453–458
Shayan R, Achen MG, Stacker SA (2006) Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis 27(9):1729–1738
Mellor RH et al (2010) Lymphatic dysfunction, not aplasia, underlies Milroy disease. Microcirculation 17(4):281–296
Connell F, Brice G, Mortimer P (2008) Phenotypic characterization of primary lymphedema. Ann N Y Acad Sci 1131:140–146
Croteau SE et al (2014) Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr 164(2):383–388
Ji Y et al (2019) Kaposiform lymphangiomatosis and kaposiform hemangioendothelioma: similarities and differences. Orphanet J Rare Dis 14(1):165
Frye M et al (2020) EphrinB2-EphB4 signalling provides Rho-mediated homeostatic control of lymphatic endothelial cell junction integrity. Elife. https://doi.org/10.7554/eLife.57732
Norden PR et al (2020) Shear stimulation of FOXC1 and FOXC2 differentially regulates cytoskeletal activity during lymphatic valve maturation. Elife. https://doi.org/10.7554/eLife.53814
Crampton SP, Davis J, Hughes CC (2007) Isolation of human umbilical vein endothelial cells (HUVEC). J Vis Exp 3:183
Mikelis C et al (2009) Integrin alpha(v)beta(3) is a pleiotrophin receptor required for pleiotrophin-induced endothelial cell migration through receptor protein tyrosine phosphatase beta/zeta. Faseb J 23(5):1459–1469
Mikelis C et al (2011) A peptide corresponding to the C-terminal region of pleiotrophin inhibits angiogenesis in vivo and in vitro. J Cell Biochem 112(6):1532–1543
Melendez J et al (2011) RhoA GTPase is dispensable for actomyosin regulation but is essential for mitosis in primary mouse embryonic fibroblasts. J Biol Chem 286(17):15132–15137
Ren XD, Kiosses WB, Schwartz MA (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 18(3):578–585
Drummond RA et al (2018) GM-CSF therapy in human caspase recruitment domain-containing protein 9 deficiency. J Allergy Clin Immunol 142(4):1334-1338.e5
Srivastava S et al (2020) Low dose of penfluridol inhibits VEGF-induced angiogenesis. Int J Mol Sci 21(3):755
Hossian A et al (2018) Multipronged activity of combinatorial miR-143 and miR-506 inhibits Lung Cancer cell cycle progression and angiogenesis in vitro. Sci Rep 8(1):10495
Carpentier G, Martinelli M, Courty J, Cascone I (2012) Angiogenesis analyzer for ImageJ. In: 4th ImageJ user and developer conference proceedings. pp 198–201
Shevchenko A et al (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1(6):2856–2860
Akwii RG et al (2021) In vivo ear sponge lymphangiogenesis assay. Methods Mol Biol 2193:85–96
Garcia-Caballero M et al (2017) Modeling pre-metastatic lymphvascular niche in the mouse ear sponge assay. Sci Rep 7:41494
Durre T et al (2018) uPARAP/Endo180 receptor is a gatekeeper of VEGFR-2/VEGFR-3 heterodimerisation during pathological lymphangiogenesis. Nat Commun 9(1):5178
Luo J et al (2017) RhoA and RhoC are involved in stromal cell-derived factor-1-induced cell migration by regulating F-actin redistribution and assembly. Mol Cell Biochem 436(1–2):13–21
Kutys ML, Yamada KM (2014) An extracellular-matrix-specific GEF-GAP interaction regulates Rho GTPase crosstalk for 3D collagen migration. Nat Cell Biol 16(9):909–917
Wilde C et al (2000) Recognition of RhoA by Clostridium botulinum C3 exoenzyme. J Biol Chem 275(22):16478–16483
Toda A et al (2015) Rho GTPase recognition by C3 exoenzyme based on C3-RhoA complex structure. J Biol Chem 290(32):19423–19432
Wilde C, Vogelsgesang M, Aktories K (2003) Rho-specific Bacillus cereus ADP-ribosyltransferase C3cer cloning and characterization. Biochemistry 42(32):9694–9702
Kim KL et al (2006) Interaction between Tie receptors modulates angiogenic activity of angiopoietin2 in endothelial progenitor cells. Cardiovasc Res 72(3):394–402
Korhonen EA et al (2016) Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest 126(9):3495–3510
Hakanpaa L et al (2015) Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat Commun 6:5962
Huttenlocher A, Horwitz AR (2011) Integrins in cell migration. Cold Spring Harb Perspect Biol 3(9):a005074
Zhao X, Guan JL (2011) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 63(8):610–615
Golubovskaya VM et al (2008) A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem 51(23):7405–7416
Brandvold KR et al (2012) Development of a highly selective c-Src kinase inhibitor. ACS Chem Biol 7(8):1393–1398
Jaffe AB, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–269
Guilluy C et al (2011) The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol 13(6):722–727
Patel M, Karginov AV (2014) Phosphorylation-mediated regulation of GEFs for RhoA. Cell Adh Migr 8(1):11–18
Matsui T et al (1996) Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. Embo J 15(9):2208–2216
Gavard J, Patel V, Gutkind JS (2008) Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14(1):25–36
Kalappurakkal JM et al (2019) Integrin mechano-chemical signaling generates plasma membrane nanodomains that promote cell spreading. Cell 177(7):1738-1756.e23
Breitsprecher D, Goode BL (2013) Formins at a glance. J Cell Sci 126(Pt 1):1–7
Kuhn S, Geyer M (2014) Formins as effector proteins of Rho GTPases. Small GTPases 5:e29513
Takeya R et al (2008) The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. Embo J 27(4):618–628
Muzumdar MD et al (2007) A global double-fluorescent Cre reporter mouse. Genesis 45(9):593–605
Basu S, Kustanovich I, Lamprecht R (2016) Arp2/3 and VASP are essential for fear memory formation in lateral amygdala. eNeuro. https://doi.org/10.1523/ENEURO.0302-16.2016
Silveira AAA et al (2018) TNF induces neutrophil adhesion via formin-dependent cytoskeletal reorganization and activation of beta-integrin function. J Leukoc Biol 103(1):87–98
Scholz A, Plate KH, Reiss Y (2015) Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci 1347:45–51
Holopainen T et al (2012) Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst 104(6):461–475
Lewis CE, Ferrara N (2011) Multiple effects of angiopoietin-2 blockade on tumors. Cancer Cell 19(4):431–433
Mazzieri R et al (2011) Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19(4):512–526
Goede V et al (2010) Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer 103(9):1407–1414
Zonneveld R et al (2017) Low serum angiopoietin-1, high serum angiopoietin-2, and high Ang-2/Ang-1 protein ratio are associated with early onset sepsis in surinamese newborns. Shock 48(6):638–643
Michalska-Jakubus M et al (2019) Imbalanced serum levels of Ang1, Ang2 and VEGF in systemic sclerosis: integrated effects on microvascular reactivity. Microvasc Res 125:103881
Li P et al (2015) Diagnostic and prognostic potential of serum angiopoietin-2 expression in human breast cancer. Int J Clin Exp Pathol 8(1):660–664
Boureux A et al (2007) Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol 24(1):203–216
Ridley AJ, Hall A (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70(3):389–399
Spindler V, Schlegel N, Waschke J (2010) Role of GTPases in control of microvascular permeability. Cardiovasc Res 87(2):243–253
Cascone I et al (2003) Tie-2-dependent activation of RhoA and Rac1 participates in endothelial cell motility triggered by angiopoietin-1. Blood 102(7):2482–2490
Mammoto T et al (2007) Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 282(33):23910–23918
Souma T et al (2018) Context-dependent functions of angiopoietin 2 are determined by the endothelial phosphatase VEPTP. Proc Natl Acad Sci USA 115(6):1298–1303
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687
Danilucci TM et al (2019) Recombinant RGD-disintegrin DisBa-01 blocks integrin alphavbeta3 and impairs VEGF signaling in endothelial cells. Cell Commun Signal 17(1):27
Takada YK et al (2017) Direct binding to integrins and loss of disulfide linkage in interleukin-1beta (IL-1beta) are involved in the agonistic action of IL-1beta. J Biol Chem 292(49):20067–20075
Hu B et al (2006) Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res 66(2):775–783
Imanishi Y et al (2007) Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res 67(9):4254–4263
Bezuidenhout L, Zilla P, Davies N (2009) Association of Ang-2 with integrin beta 2 controls Ang-2/PDGF-BB-dependent upregulation of human peripheral blood monocyte fibrinolysis. Inflammation 32(6):393–401
Thomas M et al (2010) Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J Biol Chem 285(31):23842–23849
Ruoslahti E (1996) RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715
Schmidt A, Hall A (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 16(13):1587–1609
Iwanicki MP et al (2008) FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci 121(Pt 6):895–905
Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84(3):359–369
Higgs HN (2005) Formin proteins: a domain-based approach. Trends Biochem Sci 30(6):342–353
Courtemanche N (2018) Mechanisms of formin-mediated actin assembly and dynamics. Biophys Rev 10(6):1553–1569
Alberts AS et al (1998) Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J Biol Chem 273(15):8616–8622
Scharpfenecker M et al (2005) The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118(Pt 4):771–780
Brash JT et al (2020) Tamoxifen-activated CreERT impairs retinal angiogenesis independently of gene deletion. Circ Res 127(6):849–850
Jafree DJ et al (2021) Mechanisms and cell lineages in lymphatic vascular development. Angiogenesis 24(2):271–288
Geng X et al (2020) S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling. JCI Insight. https://doi.org/10.1172/jci.insight.137652
Acknowledgements
The authors thank Dr. Guillermo Oliver (Northwestern University) for providing the Prox1-CreERT2 mice and the members of the TTUHSC animal facility in Amarillo for their support. This work was supported in part by the National Institutes of Health Grant (NCI) R15CA231339 and Texas Tech University Health Sciences Center (TTUHSC) School of Pharmacy Office of the Sciences grant. The common equipment used was obtained through the Cancer Prevention Research Institute of Texas (CPRIT) Grants RP110786, RP190524 and RP200572. The funders had no role in study design, decision to write, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, RGA and CMM; Methodology, RGA, MSS; Validation, FTZ, CMM; Investigation, RGA, MSS, FTZ, MZM and CMM; Writing—Original Draft, RGA and CMM; Writing—Review & Editing, FTZ, MSS, PT, MZM, YZ, JSG and CLD; Resources, PT, YZ, JSG and CLD; Supervision, CMM; Funding Acquisition: CMM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10456_2022_9831_MOESM1_ESM.pdf
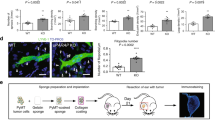
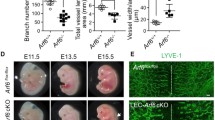
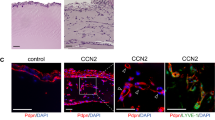
Supplementary Figure 1: Ang2 does not affect Human Umbilical Vein Endothelial Cell (HUVEC) angiogenic functions. (A) Quantification of Ang2-induced HUVEC proliferation (n = 3). (B) Quantification of Ang2-induced HUVEC migration (n = 3). (C-F) Quantification of Ang2-induced sprout formation on HUVEC, assessed by the number of nodes (C), junctions (D) and total sprout length (E), and representative images (F) (n = 3), ns: not significant. Supplementary Figure 2: Ang2 affects Human Dermal Lymphatic Endothelial Cell (HDLEC) proliferation, but not sprout formation. (A) Quantification of Ang2-induced HDLEC proliferation (n = 3). (B-E) Quantification of Ang2-induced (20 ng/ml) sprout formation on HDLEC, assessed by number of nodes (B), junctions (C) and total sprout length (D), and representative images (E) (n = 4). *P < 0.05; **P < 0.01; ns: not significant. Supplementary Figure 3. Ang2 induces Human Umbilical Vein Endothelial Cell (HUVEC) RhoA activation. (A, B) Representative images (A) and quantification (B) upon dose-response of Ang2 treatment (n = 3); ns: not significant. Supplementary Figure 4: Ang2 treatment induces Tie1 and inhibits Tie2 phosphorylation in HDLEC. (A) Representative images and (B) Quantification of Tie1 and Tie2 phosphorylation in HDLEC upon Ang2 (20 ng/ml) treatment (n = 2 for Tie1 and n = 3 for Tie2). ***P < 0.001. Supplementary Figure 5: Toxicity and efficiency evaluation of Src and FAK inhibitors. (A) Quantification of HDLEC cell number upon treatment (5 μM and 10 μΜ) with SU-6656, PP2 or PF-573 (n = 2). (B, C) Representative images of Src (B) and FAK (C) phosphorylation in the presence of PP2 (B) and PF-573 (C) inhibitors respectively (n = 3). (D, E) Quantification of Ang2-induced (20 ng/ml) cell migration upon treatment with Fasudil (10 μM) or Y-27632 (10 μΜ) at different time points (1, 6, 16 h) (F) Representative images of HDLEC with phalloidin staining (red) after treatment of Ang2 (20 ng/ml) upon Fasudil (10 μΜ) (D) or Y-27632 (10 μΜ) (E) treatment (n = 2) (blue: nuclei). *P < 0.05; ***P < 0.001; ns: not significant. Supplementary Figure 6: Toxicity evaluation of formin activator and inhibitor. (A) Quantification of HDLEC cell number upon treatment with IMM01 (10 μM and 100 μΜ) and with SMIFH2 (5 μΜ and 10 μΜ) (n = 2). *P < 0.05; **P < 0.01; ***P < 0.001; ns: not significant. Supplementary Figure 7: Evaluation of Ang2-induced lymphangiogenesis in the ear sponge assay after 21 days of sponge implantation. (A) Schematic diagram of the ear sponge assay model. (B, C) Representative images (B) and quantification (assessed as lymphatic vessel area and density) (C) of Ang2-induced lymphangiogenesis after 21 days of sponge incubation (n = 2). (D) Representative images of ear sponge stained for LYVE1 and Tie2 (n = 2). White arrows denote representative areas with colocalized signal. (E) Representative images of RhoA expression in isolated dermal lymphatic endothelial cells from tamoxifen-treated RhoAiΔLEC mice and littermate controls. Scale bars, 500 μm, 100 μm. *P < 0.05; ns: not significant. Supplementary Table 1: List of proteins interacting with Angiopoietin-2. Text in Bold shows the interaction between Ang2 and integrin beta-1 upon Ang2 treatment. Supplementary Table 2: List of proteins interacting with Tie2. Text in Bold shows the interaction between Tie2 and integrin beta-1 upon Ang2 treatment. Supplementary file1 (PDF 1505 kb)
Appendix
Appendix
Key Resources Table
Reagents or resources | Source | Identifier | |
|---|---|---|---|
Cells and media | |||
HDLEC | PromoCell | Cat# C-12216 | |
Endothelial cell growth supplement | R&D systems | Cat# CCM027 | |
Endothelial cell base growth media | R&D systems | Cat# CCM028 | |
M199 medium | Corning | Cat# MT10060CV | |
Fetal Bovine Serum | GIBCO™ | Cat# 10438026 | |
DMEM | Life Technologies Corporation | Cat# 11995073 | |
Antibodies | |||
RhoA (67B9) | Cell Signaling Technology | Cat# 2117; RRID: AB_1069392 | |
Tie1 (D2K2T) | Cell Signaling Technology | Cat# 23111; RRID: AB_2798856 | |
Tie2 | R&D Systems | Cat# AF313; RRID: AB_355295 | |
Integrin beta-1 (D6S1W) | Cell Signaling Technology | Cat# 34971; RRID: AB_2799067 | |
mDia1 (Diap1) | Cell Signaling Technology | Cat# 5486; RRID: AB_10828440 | |
FHOD1 | ECM Biosciences | Cat# FM3521; RRID: AB_2104508 | |
Angiopoietin-2 | Cell Signaling Technology | Cat# 2948; RRID: AB_2289507 | |
Angiopoietin-2 | Santa Cruz Biotechnology | Cat# sc-74403, RRID: AB_1118956 | |
Tubulin | Cell Signaling Technology | Cat# 2146; RRID: AB_2210545 | |
Actin | Cell Signaling Technology | Cat# 3700; RRID: AB_2242334 | |
GEF-H1 | Cell Signaling Technology | Cat# 4076; RRID: AB_2060032 | |
PDZ-Rho GEF | Abcam | Cat# ab110059; RRID: AB_10863676 | |
LARG | Abcam | Cat# ab136072; RRID: AB_2828035 | |
FAK | Cell Signaling Technology | Cat# 3285; RRID: AB_2269034 | |
Phospho-FAK (Tyr397) (D20B1) | Cell Signaling Technology | Cat# 8556; RRID: AB_10891442 | |
Src | Cell Signaling Technology | Cat# 2108; RRID: AB_331137 | |
Phospho-Src | Cell Signaling Technology | Cat# 2101; RRID: AB_331697 | |
Myosin light chain 2 | Cell Signaling Technology | Cat# 3672; RRID: AB_10692513 | |
Phospho-myosin light chain 2 | Cell Signaling Technology | Cat# 3674; RRID: AB_2147464 | |
Akt | Cell Signaling Technology | Cat# 9272; RRID: AB_329827 | |
Phospho-Akt (Ser473) | Cell Signaling Technology | Cat# 4060; RRID: AB_2315049 | |
PY—4G10 | Millipore | Cat# 05-1050X; RRID: AB_916370 | |
Anti-rabbit | Southern Biotech | Cat# 4010-05; RRID: AB_2632593 | |
Anti-goat | Southern Biotech | Cat# 6420-05; RRID: AB_2796335 | |
Anti-mouse | Southern Biotech | Cat# 1010-05; RRID: AB_2728714 | |
LYVE1 | R&D systems | Cat# AF2125; RRID: AB_2297188 | |
IgG mouse isotype control | Santa Cruz Biotechnology | Cat# sc-2025; RRID: AB_737182 | |
LYVE-1 | ReliaTech | Cat# 103-PA50; RRID: AB_2783787 | |
VE-cadherin | Cell Signaling Technology | Cat# 2500; RRID: AB_10839118 | |
ZO-1 | Invitrogen | Cat# 33-9100; RRID: AB_87181 | |
siRNAs | |||
RhoA 1, s758 | Ambion | Cat# 4390826 | |
RhoA 2, s759 | Ambion | Cat# 4390826 | |
Tie1 a, s14142 | Ambion | Cat# 4392420 | |
Tie1 b, s14141 | Ambion | Cat# 4392420 | |
Tie 2 a, s13984 | Ambion | Cat# 4457298 | |
Tie 2 b, s13984 | Ambion | Cat# 4390824 | |
Integrin beta 3, s7575 | Ambion | Cat# 4390824 | |
Integrin beta 1, s112581 | Ambion | Cat# AM51331 | |
mDia1 | Dharmacon | Cat# M-010347-02-0005 | |
FHOD1 | Dharmacon | Cat# M-013709-01-0005 | |
Silencer® Select Negative Control siRNA | Thermo Fisher Scientific | Cat# 4390844 | |
Other reagents & materials | |||
DharmaFECT 1 | Dharmacon | Cat# T-2001 | |
Tamoxifen | Alfa-Aesar | Cat# 10540-29-1 | |
JumpStart REDTaq Ready-Mix Reaction Mix | Millipore Sigma | Cat# P0982-100-RXN | |
Antibiotic–antimycotic solution | GIBCOTM | Cat# 15240-062 | |
Glutathione Sepharose 4B beads | GE-Healthcare | Cat# 45-000-139 | |
Protease and phosphatase inhibitor cocktail | Thermo Scientific | Cat# 1861281 | |
Immobilon Western Chemiluminescent HRP substrate | Millipore | Cat# WBKLS0500 | |
Immobilon P | Millipore | Cat# IPVH304F0 | |
Polycarbonate membranes | NeuroProbe | Cat# PFB8 | |
Trypsin–EDTA | Life Technologies | Cat# 25300-054 | |
RGF-basement membrane extract | Trevigen | Cat# 3433 | |
Qiagen’s RNeasy mini kit | Qiagen | Cat# 74101 | |
Verso cDNA synthesis kit | Thermo Scientific | Cat# AB-14531/A | |
SYBR Green PCR MasterMix | Thermo Fisher Scientific | Cat# 4309155 | |
Dyna beads A | Invitrogen | Cat# 10004D | |
Dyna beads G | Invitrogen | Cat# 10002D | |
Human Ang2 | Peprotech | Cat# 130-07 | |
Mouse Ang2 | Fisher Scientific | Cat# 7186-AN | |
C3 toxin | Fisher Scientific | Cat# NC9317720 | |
RGD | Fisher Scientific | Cat# NC0210557 | |
PP2 | Fisher Scientific | Cat# 52-957-31MG | |
PF-573228 | Fisher Scientific | Cat# 50-101-3643 | |
SU-6656 | Fisher Scientific | Cat# 57-263-51MG | |
Y-27632 | Fisher Scientific | Cat# 12-541-0 | |
Fasudil | LC Laboratories | Cat# F4660 | |
IMM01 | Sigma Aldrich | Cat# SML1064 | |
SMIFH2 | Sigma Aldrich | Cat# S4826 | |
Dispase | Fisher Scientific | Cat# 17105-041 | |
Collagenase Type II | Fisher Scientific | Cat# 17101-015 | |
qPCR oligonucleotides (Primers) | |||
Human RhoA Forward: 5′-AGCCAAGATGAAGCAGGAGC-3′ | Integrated DNA Technologies | ||
Human RhoA Reverse: 5′-TTCCCACGTCTAGCTTGCAG-3′ | Integrated DNA Technologies | ||
Human Ang2 Forward: 5′-AAGAGAAAGATCAGCTACAGG-3′ | Integrated DNA Technologies | ||
Human Ang2 Reverse: 5′- CCTTAGAGTTTGATGTGGAC-3′ | Integrated DNA Technologies | ||
Human Actin Forward: 5′-CTCTTCCAGCCTTCCTTCCTG-3′ | Integrated DNA Technologies | ||
Human Actin Reverse: 5′- CAGCACTGTGTTGGCGTACAG-3′ | Integrated DNA Technologies | ||
Genotyping oligonucleotides (Primers) | |||
RhoAf/f Forward: 5′-TCTCTGCACTGAGGGAGTTAGG-3′ | Integrated DNA Technologies | ||
RhoAf/f Reverse: 5′-GTACATACAGGGAATGGAAACAAGG-3′ | Integrated DNA Technologies | ||
Tom-GFP Wt Forward: 5′-CTCTGCTGCCTCCTGGCTTCT-3′ | Integrated DNA Technologies | ||
Tom-GFP Wt Reverse: 5′-CGAGGCGGATCACAAGCAATA-3′ | Integrated DNA Technologies | ||
Tom-GFP Mut Reverse: 5′-TCAATGGGCGGGGGTCGTT-3′ | Integrated DNA Technologies | ||
Prox1-CreERT2 Forward: 5′-AACTCGAGCTCTTTCTCTCTACAGTTCAACAGATGCATTACC-3′ | Integrated DNA Technologies | ||
Prox1-CreERT2 Reverse: 5′-GGGGGAGGGAGAGGGGCGGAATTGCTACTCGTGAAGGAGTTC-3′ | Integrated DNA Technologies | ||
Rights and permissions
About this article
Cite this article
Akwii, R.G., Sajib, M.S., Zahra, F.T. et al. Angiopoietin-2-induced lymphatic endothelial cell migration drives lymphangiogenesis via the β1 integrin-RhoA-formin axis. Angiogenesis 25, 373–396 (2022). https://doi.org/10.1007/s10456-022-09831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-022-09831-y