Abstract
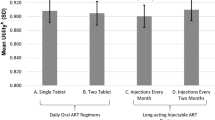
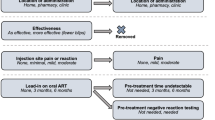
With long-acting injectable antiretroviral therapy likely to be a treatment option for people living with HIV (PLWH), it is critical to assess its acceptability among potential end-users. Based on formative qualitative work and our own ongoing development of targeted long-acting products in nanosuspension formulations, we created eight hypothetical medication scenarios varying along six dichotomous attributes: administration location (home versus [vs.] clinic), dosing frequency (every 2 weeks vs. 1 week), injections per dose (one vs. two), injection pain (mild vs. moderate), injection site reaction (mild vs. moderate), and effectiveness (better vs. same as pills). PLWH from three outpatient care clinics in Seattle, WA and Riverside, CA rated acceptability (i.e., willingness to try each hypothetical medication) from 0 (very unlikely) to 100 (very likely). In conjoint analyses, we examined level and correlates of acceptability, the impact of each attribute on overall acceptability, and moderators of this effect. Participants (median age 52 years; 71% male, 34% White, 36% Black/African American, 20% Hispanic) rated acceptability of the 8 scenarios from 47.8 (standard deviation [SD] = 37.0) to 68.8 (SD = 34.1), with effectiveness (impact score = 7.3, SD = 18.7, p = 0.005) and dosing frequency (impact score = 5.7, SD = 19.6, p = 0.034) the only attributes with a significant impact on acceptability. There were no statistically significant differences in overall acceptability according to any participant socio-demographic or other characteristic; however, gender, education, employment status, and experience with and hatred/avoidance of injections moderated some effects. Overall acceptability for targeted long-acting antiretroviral treatment as proposed was modest, with superior effectiveness and lower dosing frequency most impactful on acceptability. Future acceptability research should continue to evaluate specific products in development with a full range of conjoint analytic and other techniques.
Similar content being viewed by others
References
World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015.
AIDS Info. Guidelines for the use of antiretroviral agents in HIV-1-Infected adults and adolescents, https://aidsinfo.nih.gov/guidelines. 2018; Accessed 30 Oct 2018.
Henry J Kaiser Family Foundation. HIV viral suppression rate in US lowest among comparable high income countries, https://www.kff.org/hivaids/slide/hiv-viral-suppression-rate-in-u-s-lowest-among-comparable-high-income-countries/. 2019; Accessed 26 Apr 2019.
Nance RM, Delaney JC, Simoni JM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Ann Intern Med. 2018;169(6):376–84.
Kanters S, Park JJ, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e31–40.
Mbuagbaw L, Sivaramalingam B, Navarro T, et al. Interventions for enhancing adherence to antiretroviral therapy (ART): a systematic review of high quality studies. AIDS Patient Care STDs. 2015;29(5):248–66.
Gulick RM, Flexner C. Long-acting HIV drugs for treatment and prevention. Annu Rev Med. 2019;70:137–50.
Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–510.
Swindels S, Andrade-Villanueva J-F, Richmond GJ, et al. Long-acting cabotegravir + rilpivirine as maintenance therapy: Atlas Week 48 results. In: 2019 Conference on Retroviruses and Opportunistic Infections, Seattle, WA, March 4-7, 2019. Abstract 139.
Orkin C, Arasteh K, Hernandez-Mora MG, et al. Long-acting cabotegravir + rilpivirine for HIV maintenance: FLAIR week 48 results. In: 2019 Conference on Retroviruses and Opportunistic Infections, Seattle, WA, March 4–7, 2019. Abstract 140LB.
McConnachie LA, Kinman LM, Koehn J, et al. Long-acting profile of 4 drugs in 1 anti-HIV nanosuspension in nonhuman primates for 5 weeks after a single subcutaneous injection. J Pharm Sci. 2018;107(7):1787–90.
Koehn J, Iwamoto JF, Kraft JC, et al. Extended cell and plasma drug levels after one dose of a three-in-one nanosuspension containing lopinavir, efavirenz, and tenofovir in nonhuman primates. AIDS. 2018;32(17):2463–7.
Kraft JC, McConnachie LA, Koehn J, et al. Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma. AIDS. 2017;31(6):765.
Orme B. Getting Started with Conjoint Analysis. Madison: Research Publishers LLC; 2006.
Green P, Srinivasan V. Conjoint analysis in marketing: new developments with implications for research and practice. J Mark Res. 1990;54:3–19.
Beusterien KM, Dziekan K, Flood E, et al. Understanding patient preferences for HIV medications using adaptive conjoint analysis: feasibility assessment. Value Health. 2005;8(4):453–61.
Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. 2002;37:1681–705.
Lee SJ, Brooks R, Bolan RK, et al. Assessing willingness to test for HIV among men who have sex with men using conjoint analysis, evidence for uptake of the FDA-approved at-home HIV test. AIDS Care. 2013;25:1592–8.
Bristow CC, Lee SJ, Severe L, et al. Attributes of diagnostic tests to increase uptake of dual testing for syphilis and HIV in Port-au-Prince, Haiti. Int J STD AIDS. 2017;28:259–64.
Holt BY, Morwitz VG, Ngo L, et al. Microbicide preference among young women in California. J Womens Health. 2006;15:281–94.
Kinsler JJ, Cunningham WE, Nureña CR, et al. Using conjoint analysis to measure the acceptability of rectal microbicides among men who have sex with men in four South American cities. AIDS Behav. 2012;16(6):1436–47.
Newman PA, Duan N, Lee SJ, et al. HIV vaccine acceptability among communities at risk: the impact of vaccine characteristics. Vaccine. 2006;24(12):2094–101.
Opuni M, Bishai D, Gray GE, et al. Preferences for characteristics of antiretroviral therapy provision in Johannesburg, South Africa: results of a conjoint analysis. AIDS Behav. 2010;14:807–15.
Pines HA, Strathdee SA, Hendrix CW, et al. Oral and vaginal HIV pre-exposure prophylaxis product attribute preferences among female sex workers in the Mexico-US border region. Int J STD AIDS. 2019;30(1):45–55.
Galea JT, Kinsler JJ, Salazar X, et al. Acceptability of pre-exposure prophylaxis as an HIV prevention strategy: barriers and facilitators to pre-exposure prophylaxis uptake among at-risk Peruvian populations. Int J STD AIDS. 2011;22:256–62.
Beusterien KM, Dziekan K, Schrader S, et al. Patient preferences among third agent HIV medications: a US and German perspective. AIDS Care. 2007;19:982–8.
Simoni JM, Beima-Sofie KJM, Mohamed ZH, et al. Long-acting injectable antiretroviral treatment acceptability and preferences: A qualitative study among U.S. providers, adults living with HIV, and parents of youth living with HIV. AIDS Patient Care STDs. 2019;33(3):104–11.
Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–13.
Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrika. 1946;33(4):305–25.
Shrestha R, Karki P, Altice FL, et al. Measuring acceptability and preferences for implementation of pre-exposure prophylaxis (PrEP) using conjoint analysis: an application to primary HIV prevention among high risk drug users. AIDS Behav. 2018;22(4):1228–38.
Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, IJzerman MJ, Bridges JF. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–15.
Louviere JJ, Lancsar E. Choice experiments in health: the good, the bad, the ugly and toward a brighter future. Health Econ Policy Law. 2009;4(4):527–46.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics. 2008;26(8):661–77.
Lancsar E, Swait J. Reconceptualising the external validity of discrete choice experiments. Pharmacoeconomics. 2014;32(10):951–65.
Kim HY, Dowdy DW, Martinson NA, Kerrigan D, Tudor C, Golub J, Bridges JF, Hanrahan CF. Maternal motivation to take preventive therapy in antepartum and postpartum among HIV-positive pregnant women in South Africa: a choice experiment. AIDS Behav. 2019;23(7):1689–97.
Gazzard B, Ali S, Muhlbacher A, Ghafouri N, Maggiolo F, Golics C, Nozza S, Jose Fuster M, Antela A, Jacques Parienti J, Dang N. Patient preferences for characteristics of antiretroviral therapies: results from 5 European countries. J Int AIDS Soc. 2014;17(4 Suppl 3):19540.
Kruk ME, Riley PL, Palma AM, Adhikari S, Ahoua L, Arnaldo C, Belo DF, Brusamento S, Cumba LI, Dziuban EJ, El-Sadr WM. How can the health system retain women in HIV treatment for a lifetime? A discrete choice experiment in Ethiopia and Mozambique. PLoS ONE. 2016;11(8):e0160764.
Miners AH, Llewellyn CD, Cooper VL, Youssef E, Pollard AJ, Lagarde M, Sabin C, Nixon E, Sachikonye M, Perry N, Fisher M. A discrete choice experiment to assess people living with HIV’s (PLWHIV’s) preferences for GP or HIV clinic appointments. Sex Transm Infect. 2017;93(2):105–11.
Michaels-Igbokwe C, Lagarde M, Cairns J, Terris-Prestholt F. Designing a package of sexual and reproductive health and HIV outreach services to meet the heterogeneous preferences of young people in Malawi: results from a discrete choice experiment. Health Econ Rev. 2015;5(1):9.
Pan SW, Durvasula M, Ong JJ, Liu C, Tang W, Fu H, Wei C, Wang C, Terris-Prestholt F, Tucker JD. No place like home? Disentangling preferences for HIV testing locations and services among men who have sex with men in China. AIDS Behav. 2019;23(4):847–59.
Cameron MP, Newman PA, Roungprakhon S, Scarpa R. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine. 2013;31(36):3712–7.
Newman PA, Cameron MP, Roungprakhon S, Tepjan S, Scarpa R. Acceptability and preferences for hypothetical rectal microbicides among a community sample of young men who have sex with men and transgender women in Thailand: a discrete choice experiment. AIDS Behav. 2016;20(11):2588–601.
Quaife M, Eakle R, Cabrera Escobar MA, Vickerman P, Kilbourne-Brook M, Mvundura M, Delany-Moretlwe S, Terris-Prestholt F. Divergent preferences for HIV prevention: a discrete choice experiment for multipurpose HIV prevention products in South Africa. Med Decis Mak. 2018;38(1):120–33.
Kuteesa MO, Quaife M, Biraro S, Katumba KR, Seeley J, Kamali A, Nakanjako D. Acceptability and predictors of uptake of anti-retroviral pre-exposure prophylaxis (prep) among fishing communities in Uganda: a cross-sectional discrete choice experiment survey. AIDS Behav. 2019;23:1–3.
Dubov A, Ogunbajo A, Altice FL, Fraenkel L. Optimizing access to PrEP based on MSM preferences: results of a discrete choice experiment. AIDS Care. 2019;31(5):545–53.
van der Straten A. Placebo use influences acceptability! Findings from TRIO & QUATRO studies. In: Meeting on behavioral aspects of la and extended delivery and treatment regimens, Rockville, May 13, 2019.
Flexner C. Antiretroviral implants for treatment and prevention of Hiv infection. Curr Opin HIV AIDS. 2018;13(4):374–80.
Acknowledgements
We gratefully acknowledge the participation of clinic patients as well as the assistance with recruitment from the clinic staff. This research was supported by NIH Grants AI120176 and AI027757. SMG was supported by the Robert W. Anderson Endowed Professorship in Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simoni, J.M., Tapia, K., Lee, SJ. et al. A Conjoint Analysis of the Acceptability of Targeted Long-Acting Injectable Antiretroviral Therapy Among Persons Living with HIV in the U.S.. AIDS Behav 24, 1226–1236 (2020). https://doi.org/10.1007/s10461-019-02701-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-019-02701-7