Abstract
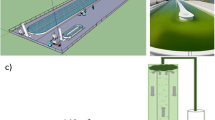
In the present study, the mass production of the rotifer species Brachionus plicatilis was evaluated using closed outdoor algae culture systems in 450- and 1000-L polyethylene bags fed with the microalgae Nannochloropsis gaditana and supplemented with probiotic bacteria Pseudoalteromonas sp. (SLP1). These bacteria were previously isolated from the digestive tract of the commercially important fish Yellowtail kingfish (Seriola lalandi, Family: Carangidae) and their stimulatory activity on growth in the microalga N. gaditana was verified. In each treatment, a significantly greater production of organisms (P < 0.05) was shown when fed with the microalgae and supplemented with beneficial bacteria over 9 days compared to control treatments without the addition of beneficial bacteria. Using denaturing gradient gel rlectrophoresis analysis (DGGE), the permanence of the SPL1 bacteria was demonstrated in a B. plicatilis culture in a 1000-L outdoor system when they were incorporated into the diet. With these results, it is shown that the use of this microalgae-bacteria mixture is feasible to improve rotifer production in outdoor batch culture systems. This is a potential input vector for beneficial bacteria as well as nutritional elements into the digestive tract of larval and juvenile stages of fish of economic importance, especially S. lalandi.





Similar content being viewed by others
References
Adarme-Vega T, Lim D, Timmins M, Vernen F, Li Y, Schenk P (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microbl Cell Fact 11:96
Ahmad A, Yamasaki S, Hirata H (1991) Optimun feeding rate of rotifer Brachionus plicatilis on the marine alga Nannochloropsis sp. J World Aquacult Soc 22(4):230–234
Avendaño R, Riquelme C (2003) Interacción bacteria-microalga en el ambiente marino y uso potencial en acuicultura. Rev Chil Hist Nat 76:725–736
Bentley CD, Carrol PM, Watanabe WO (2008) Intensive rotifer production in a pilote scale continuous culture recirculating system using nonviable microalgae and an ammonia neutralizer. J World Aquacult Soc 39(5):625–635
Brosius J, Dull TJ, Sleeter DD, Noller HF (1981) Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol 148:107–127
Brown M, Skabo S, Wilkinson B (1998) The enrichment and retention of ascorbic acid in rotifers fed microalgal diets. Aquac Nutr 4:151–156
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8(7):1137–1144
Cho DH, Ramanan R, Heo J, Lee J, Kim BH, Oh HM, Kim HS (2015) Enhancing microalgal biomass productivity by engineering a microalgal-bacterial community. Bioresour Technol 175:578–585
Conceição L, Yúfera M, Makridis P, Morais S, Dinis M (2010) Live feeds for early stages of fish rearing. Aquac Res 41:613–640
Cooper MB, Smith AG (2015) Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr Opin Plant Biol 26:147–153
Croft MT, Lawrence AD, Raux-Deery E, Warren JM, Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438:90–93
del Castillo C, Wahid M, Yoshikawa T, Sakata T (2008) Isolation and inhibitory effect of anti-Vibrio substances from Pseudoalteromonas sp. A1-J11 isolated from the coastal sea water of Kagoshima Bay. Fisheries Sci 74:174–179
Desriac F, Le Chevalier P, Brillet B, Leguerinel I, Thuillier B, Paillard C, Fleury Y (2013) Exploring the halogenome concept in marine bivalvia: haemolymph microbiota as pertinent source of probiotics for aquaculture. FEMS Microbiol Lett 350:107–116
Dhert P, Rombaut G, Suantika G, Sorgeloos P (2001) Advancement of rotifer culture and manipulation techniques in Europe. Aquaculture 200:129–146
Douillet PA (2000a) Bacterial additives that consistently enhance rotifer growth under synxenic culture conditions. 1. Evaluation of commercial products and pure isolates. Aquaculture 182:249–260
Douillet PA (2000b) Bacterial additives that consistently enhance rotifer growth under synxenic culture conditions 2. Use of single and multiple bacterial probiotics. Aquaculture 182:241–248
Dufourcq R, Chalkiadakis E, Fauchon M, Deslandes E, Kerjean V, Chanteau S, Petit E, Guezennec J, Dupont-Rouzeyrol M (2013) Isolation and partial characterization of bacteria (Pseudoalteromonas sp.) with potential antibacterial activity from a marine costal environment from New Caledonia. Lett Appl Microbiol 58(2):102–108
Epp RW, Winston PW (1978) The effects of salinity and pH on the activity and oxygen consumption of Brachionus plicatilis (Rotatoria). Comp Biochem Phys A 59A:9–12
Ferreira M, Maseda A, Fábregas J, Otero A (2008) Enriching rotifers with “premium” microalgae. Isochrysis aff. galbana clone T-ISO. Aquaculture 279:126–130
Ferreira M, Coutinho P, Seixas P (2009) Enriching rotifers with ‘premium’ microalgae Nannochloropsis gaditana. Mar Biotechnol 31:585–595
Fielder DS, Purser GJ, Battaglene SC (2000) Effect of rapid changes in temperature and salinity on availability of the rotifers Brachionus rotundiformis and Brachionus plicatilis. Aquaculture 189:85–99
Fjellheim AJ, Klinkenberg G, Skjermo J, Aasen IM, Vadstein O (2010a) Selection of candidate probionts by two different screening strategies from Atlantic cod (Gadus morhua L.) larvae. Vet Microbiol 144:153–159
Fjellheim AJ, Klinkenberg G, Skjermo J, Aasen IM, Vadstein O (2010b) Selection of candidate probionts by two different screening strategies from Atlantic cod (Gadus morhua L.) larvae. Vet Microbiol 144:153–159
Gatesoupe FJ (1991) The effect of three strains of lactic bacteria on the production rate of rotifers, Brachionus plicatilis, and their dietary value for larval turbot, Scophthalmus maximus. Aquaculture 96:335–342
Goulden EF, Hall MR, Pereg L, Baillie BK, Høj L (2013) Probiont niche specialitation contributes to additive protection against Vibrio owensii in spiny lobstyer larvae. Env Microbiol Rep 5(1):39–48
Gram L, Melchiorsen J, Bruhn J (2010) Antibacterial activity of marine culturable bacteria collected from a global sampling of ocean surface waters and surface swabs of marine organisms. Mar Biotechnol 12(4):439–451
Haché R, Plante S (2011) The relationship between enrichment, fatty acid profiles and bacterial load in cultured rotifers (Brachionus plicatilis L-strain) and Artemia (Artemia salina strain franciscana). Aquaculture 311 (1–4):201–208
Hagiwara A, Hamada K, Nishi A, Imaizumi K, Hirayama K (1993) Mass production of rotifer Brachionus plicatilis resting eggs in 50 m3 tanks. Nippon Suisan Gakk 59:93–98
Hai NV (2015) The use of probiotics in aquaculture. Review article. J Appl Microbiol 119:917–935
Hamre K (2016) Nutrient profiles of rotifers (Brachionus sp.) and rotifer diets from four different marine fish hatcheries. Aquaculture 450:136–142
Helliwell KE, Wheeler GL, Leptos KC, Goldstein RE, Smith AG (2011) Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol. Biol. Evol 28: 2921–2933
Hirata H, Murata O, Yamada S, Ishitani H, Wachi M (1998) Probiotic culture of the rotifer Branchionus plicatilis. Hydrobiologia 387(388):495–498
Hirayama K, Kunsano T (1972) Fundamental studies on physiology rotifer for its mass culture II. Influence of wáter temperatura on population growth of rotifer. Bulletin of the Japanese Society for the Science of Fish 38:1357–1363
Hoshida H, Ohira T, Minematsu A, Akada R, Nishizawa Y (2005) Accumulation of eicosapentaenoic acid in Nannochloropsis sp. in response to elevated CO2 concentrations. J Appl Phycol 17:29–34
Iehata S, Valenzuela F, Riquelme C (2013) Analysis of bacterial community and bacterial nutritional enzyme activity associated with the digestive tract of wild Chilean octopus (Octopus mimus Gould, 1852). Aquac Res 46:861–873
Isnansetyo A, Istiqomah I, Muhtadi SS, Hernawan RK, Triyanto WJ (2009) A potential bacterial biocontrol agent, strain S2V2 against pathogenic marine Vibrio in aquaculture. World J Microb Biot 25:1103–1113
Jin G, Wang SX-H (2010) Identification of a marine antagonistic strain JG1 and stablishment of a polymerase chain reaction detection technique base don the gyrB gene. Aquac Res 41:1867–1874
Karlson B, Potter D, Kuylenstierna M, Andersen RA (1996) Ultrastructure, pigment composition, and 18S rRNA gene sequence for Nannochloropsis granulata sp. nov. (Monodopsidaceae, Eustigmatophyceae), a marine ultraplankter isolated from the Skagerrak, northeast Atlantic Ocean. Phycologia 35:253–260
Kazamia E, Czesnick H, Nguyen TT, Croft MT, Sherwood E, Sasso S, Hodson SJ, Warren MJ, Smith AG (2012) Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol 14:1466–1476
Kesarcodi-Watson A, Miner P, Nicolas J, Asmani K, Robert R (2016) Pathogenic threats and probiotic use in larviculture of the scallop, Pecten maximus. Aquac Res 47:1221–1230
Kim BH, Ramanan R, Cho DH, Oh HM, Kim HS (2014) Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenergy 69:95–105
Kobayashi T, Nagase T, Hino A, Takeuchi T (2008) Effect of combination feeding of Nannochloropsis and freshwater Chlorella on the fatty acid composition of rotifer Brachionus plicatilis in a continuous culture. Fisheries Sci 74:649–656
Kostopoulou V, Vadstein O (2007) Growth performance of the rotifers Brachionus plicatilis, B. ‘Nevada’ and B. ‘Cayman’ under different food concentrations. Aquaculture 273:449–458
Kostopoulou V, Vasilakis M, Divanach P (2012) Semi-continuous mass culture of rotifers (Brachionus plicatilis) using an automatic feeder. Aquac Res 43:91–98
Kouzuma A, Watanabe K (2015) Exploring the potential of algae/bacteria interactions. Curr Opin Biotech 33:125–129
Lavens P, Sorgeloos P (1996) Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper No. 361. Food And Agriculture Organization of the United Nations, Rome, Italy
Leyton Y, Sayes C, Mejias C, Abarca M, Wilson R, Riquelme C (2017) Increased larval survival of Seriola lalandi using Pseudoalteromonas sp. as probiotics. Rev Biol Mar Oceanog 52(1):95–101
Loo P, Chong V, Vikineswary S, Ibrahim S (2015) Waste-grown phototrophic bacterium supports culture of the rotifer, Brachionus rotundiformis. Aquac Res 47:3029–3041
López R, Monteón V, González E, Montejo R, Monteón Y, Chan M (2012) Isolation and assessment of the crude extract antimicrobial activity of marine bacterium Pseudoalteromonas sp. Rev Mex Cienc Farm 43(4):38–46
Lubzens E, Zmora O (2003) Production and nutritional value of rotifers. In Live feeds in marine aquaculture (ed. by Støttrupm J G., McEvoy L A), pp. 17–64. Blackwell Publishing, Oxford, UK
Mæhre HK, Hamre K, Elvevoll EO (2013) Nutrient evaluation of rotifers and zooplankton: feed for marine fish larvae. Aquacult Nut 19:301–311
Malekzadeh Viayeh R, Mohammadi H, Banj Shafiei A (2010) Population growth of six Iranian Brachionus rotifer strains in response to salinity and food type. Int Rev Hydrobiol 95(6):461–470
Matsunari H, Hashimoto H, Oda K, Masuda Y, Imaizumi H, Teruya K, Furuita H, Yamamoto T, Hamada K, Mushiake K (2012) Effect of different algae used for enrichment of rotifers on growth, survival, and swim bladder inflation of larval amberjack Seriola dumerili. Aquacult Int 20:981–992
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E (2010) Review: the current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18
Mert Eryalçın K, Ganuza E, Atalah E, Hernández Cruz MC (2015) Nannochloropsis gaditana and Crypthecodinium cohnii, two microalgae as alternative sources of essential fatty acids in early weaning for gilthead seabream. Hidrobiológica 25(2):193–203
Montagnes DJS, Kimmance SA, Tsounis G, Gumbs JC (2001) Combined effect of temperature and food concentration on grazing rate of the rotifer Brachionus plicatilis. Mar Biol 139:975–979
Morya VK, Choi W, Kim E (2014) Isolation and characterization of Pseudoalteromonas sp. from fermented Korean food, as an antagonist to Vibrio harveyi. Appl Biochem Biotechnol 172(7):3390–3401
Murillo I, Villamil L (2011) Bacillus cereus and Bacillus subtilis used as probiotics in rotifer (Brachionus plicatilis) cultures. J Aquac Res Development S1:007. https://doi.org/10.4172/2155-9546.S1-007
Natrah F, Bossier P, Sorgeloos P, Yusoff F, Defoirdt T (2014) Significance of microalgal-bacterial interactions for aquaqculture. Rev Aquacult 6:48–61
Nordgreen A, Penglase S, Hamre K (2013) Increasing the levels of the essential trace elements Se, Zn, Cu and Mn in rotifers (Brachionus plicatilis) used as live feed. Aquaculture 380-383:120–129
Olsen Y (2004) Live food technology of cold-water marine fish larvae. in Culture of cold-water marine fish (ed. by E. Mokness, E. Kjrsvik and Y. Olsen), pp.73–128. Blackwell Publishing, Oxford, UK
Önal U, Çelik İ, Ergün S (2010) The performance of a small-scale, high-density, continuous system for culturing the rotifer Brachionus plicatilis. Turk J Fish Aquat Sci 34(2):187–195
Otero A, García D, Fabregas J (1997) Factors controlling eico-sapentaenoic acid production in semicontinuous cultures of marine microalgae. J Appl Phycol 9:465–469
Pandiyan P, Balaraman D, Thirunavukkarasu R, Gnana E, Subaramaniyan K, Manikkan S, Sadayappan B (2013) Probiotics in aquaculture. Review article. Drug Invention Today 5:55–59
Pham D, Ansquer D, Chevalier A, Dauga C, Peyramale A, Wabete N, Labreuche Y (2014) Selection and characterization of potential probiotic bacteria for Litopenaeus stylirostris shrimp hatcheries in New Caledonia. Aquaculture 432:475–482
Planas M, Vázquez JA, Marques J, Pérez-Lomba R, González MP, Murado M (2004) Enhancement of rotifer (Brachionus plicatilis) growth by using terrestrial lactic acid bacteria. Aquaculture 240:313–329
Plaza J, Leyton Y, Sayes C, Mejias C, Riquelme C (2017) Seriola lalandi larviculture with probiotic supplements in mesocosm systems. J FisheriesSciences.com 11(3): 64-70
Prol-Garcıa M J, Planas M, Pintado J (2010) Different colonization and residence time of Listonella anguillarum and Vibrio splendidus in the rotifer Brachionus plicatilis determined by real-time PCR and DGGE. Aquaculture 302: 26–35
Qi Z, Dierckens K, Defoirdt T, Sorgeloos P, Boon N, Bao Z, Bossier P (2009) Effects of feeding regime and probionts on the diverting microbial communities in rotifer Brachionus culture. Aquacult Int 17:303–315
Rajendiran A, Subramanian P (2007) Mass production of freshwater rotifer Brachionus calyciflorus, under different diets and regimes. J Appl Aquac 19(3):101–111
Ramanan R, Kim B-H, Cho D-H, Oh H-M, Kim H-S (2016) Algae–bacteria interactions: evolution, ecology and emerging applications. Research review paper Biotechnol Adv 34:14–29
Reguera B (1984) The effect of ciliate contamination in mass cultures of the rotifer, Brachionus plicatilis O.F. Müller. Aquaculture 40(2):103–108
Rehberg-Haas S, Meyer S, Lippemeier S, Schulz C (2015) A comparison among different Pavlova sp. products for cultivation of Brachionus plicatilis. Aquaculture 435:424–430
Rekiel A, Wiecek J, Bielecki W, Gajewska J, Cichowicz M, Kulisiewicz J, Batorska M, Roszkowski T et al (2007) Effect of addition of feed antibiotic flavomycin or prebiotic BIO-MOS on production results of fatteners, blood biochemical parameters, morphometric indices of intestine and composition of microflora. Archiv Tierzucht Dummerstorf 50:172–180
Rigos G, Smith P (2015) A critical approach on pharmacokinetics, pharmacodynamics, dose optimisation and withdrawal times of oxytetracycline in aquaculture. Rev Aquac 7:77–106
Riquelme C, Araya R, Escribano R (2000) Selective incorporation of bacteria by Argopecten purpuratus larvae: implications for the use of probiotics in culturing systems of the Chilean scallop. Aquaculture 181:25–36
Rocha JM, Garcia JE, Henríquez MH (2003) Growth aspects of marine microalga Nannochloropsis gaditana. Biomol Eng 20:237–242
Rodolfi L, Zittelli GC, Barsanti L, Rosati G, Tredici M (2013) Growth medium recycling in Nannochloropsis sp. mass cultivation. Biomol Eng 20:243–248
Rombaut G, Grommen R, Zizhong Q, Vanhoof V, Suantika G, Dhert P, Sorgeloos P, Verstraete W (2003) Improved performance of an intensive rotifer culture system by using a nitrifying inoculum (ABIL). Aquac Res 34:165–174
Rumí Pastor A (2007) Control system for rotifer production. Trondheim, August 2007. Master's thesis. NTNU. Norwegian University of Science and Technology
Sánchez-Torres H, Juscamaita-Morales J, Vargas-Cárdenas J, Oliveros-Ramos R (2008) Producción de la microalga Nannochloropsis oculata (Droop) Hibberd en medios enriquecidos con ensilado biológico de pescado. Ecol Appl 6: 97–100
Sayegh F, Radi N, Montagnes D (2007) Do strain differences in microalgae alter their relative quality as afood for the rotifer Brachionus plicatilis? Aquaculture 273:665–678
Sayes C, Leyton Y, Riquelme C (2016) Bacteria Pseudoaltermonas sp. con potencial probiótico para cultivos larvales de peces. Lat Am J Aquat Res 44(1):76–84
Seychelles L, Audet C, Tremblay R, Fournier R, Pernet F (2009) Essential fatty acid enrichment of cultured rotifers (Brachionus plicatilis, Müller) using frozen-concentrated microalgae. Aquacult Nut 15:431–439
Sherman PM, Ossa JC, Johnson-Henry K (2009) Unraveling mechanisms of action of probiotics. Nutr Clin Pract 24(1):10–14
Shiri Harzevili A R, Van Duffel H, Dhert Ph, Swings J, Sorgeloos P (1998) Use of potential probiotic Lactococcus lactis AR21 strain for the enhancement of growth in the rotifer Brachionus plicatilis (Müller). Aquac Res 29: 411–417
Skjermo J, Bakke I, Dahle SW, Vadstein O (2015) Probiotic strains introduced through live feed and rearing water have low colonizing success in developing Atlantic cod larvae. Aquaculture 438:17–23
Sukenik A (1991) Ecophysiological considerations in optimization of eicosapentaenoic production by Nannochloropsis sp. (Eustigmatophyceae). Bioresour Technol 35:263–370
Tamaru CS, Lee CS (1991) Improving the larval rearing of striped mullet (Mugil cephalus) by manipulating quantity and quality of the rotifer Brachionus plicatilis. In: Proceeding of US–Asia workshop. The Oceanic Institute, Honolulu, Hawaii, pp. 79–88
Teplitski M, Rajamani S (2011) Signal and nutrient exchange in the interactions between soil algae and bacteria. In: Biocommunication in soil microorganisms; Witzany, G., Ed.; Springer: Berlin, Germany, 2011; pp. 413–426
Toi H T, Boeckx P, Sorgeloos P, Bossier P, Van Stappen G (2014) Co-feeding of microalgae and bacteria may result in increased N assimilation in Artemia as compared to mono-diets, as demonstrated by a 15N isotope uptake laboratory study. Aquaculture 422–423: 109–114
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Molr Biol R 64:655–671
Villamil LM, Infante SM, Lecompte OP (2012) Uso de microorganismos benéficos en el alimento vivo para controlar la aparición de enfermedades durante el cultivo de animales acuáticos. Revista Mutis 2(2):89–106
Wullur S, Sakakura Y, Hagiwara A (2009) The minute monogonont rotifer Proales similis de Beauchamp: culture and feeding to small mouth marine fish larvae. Aquaculture 293:62–67
Zink I, Douillet P, Benett D (2013) Improvement of rotifer Brachionus plicatilis population growth dynamics with inclusión of Bacillus spp. probiotics. Aquac Res 44:200–211
Acknowledgments
This study was financed by the Fund for Promotion of Scientific and Technological Development, of the Chilean National Science Foundation (FONDEF, CONICYT) Grant No. IT13I20008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mejias, C., Riquelme, C., Sayes, C. et al. Production of the rotifer Brachionus plicatilis (Müller 1786) in closed outdoor systems fed with the microalgae Nannochloropsis gaditana and supplemented with probiotic bacteria Pseudoalteromonas sp. (SLP1). Aquacult Int 26, 869–884 (2018). https://doi.org/10.1007/s10499-018-0253-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0253-3