Abstract
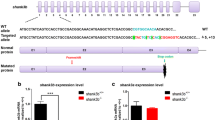
Fragile X syndrome (FXS) is a heritable mental retardation disease caused by unstable trinucleotide repeat sequences in FMR1. FXS is characterized by delayed development, hyperactivity, and autism behavior. Zebrafish is an excellent model to study FXS and the underlying function of fmr1. However, at present, fmr1 function is mainly studied via morpholinos or generated mutants using targeting induced local lesions in genomes. However, both of these methods generate off-target effects, making them suboptimal techniques for studying FXS. In this study, CRISPR/Cas9 technology was used to generate two zebrafish fmr1 mutant lines. High-throughput behavior analysis, qRT-PCR, and alcian blue staining experiments were employed to investigate fmr1 function. The fmr1 mutant line showed abnormal behavior, learning memory defects, and impaired craniofacial cartilage development. These features are similar to the human FXS phenotype, indicating that the fmr1 mutant generated in this study can be used as a new model for studying the molecular pathology of FXS. It also provides a suitable model for high-throughput screening of small molecule drugs for FXS therapeutics.




Similar content being viewed by others
References
Arbab T, Pennartz CMA, Battaglia FP (2018) Impaired hippocampal representation of place in the Fmr1-knockout mouse model of fragile X syndrome. Sci Rep 8:8889
Ashley CT Jr, Wilkinson KD, Reines D, Warren ST (1993) Fmr1 protein: conserved RNP family domains and selective RNA binding. Science 262:563–566
Belmonte MK, Bourgeron T (2006) Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci 9:1221–1225
Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438(7070):1017–1021
Davis JK, Broadie K (2017) Multifarious functions of the fragile X mental retardation protein. Trends Genet 33:703–714
De Diego Otero Y, Severijnen LA, van Cappellen G, Schrier M, Oostra B, Willemsen R (2002) Transport of fragile X mental retardation protein via granules in neurites of PC12 cells. Mol Cell Biol 22:8332–8341.
den Broeder MJ, van der Linde H, Brouwer JR, Oostra BA, Willemsen R, Ketting RF (2009) Generation and characterization of FMR1 knockout zebrafish. PLoS ONE 4:e7910
Draper BW, Morcos PA, Kimmel CB (2001) Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis 30:154–156
El-Brolosy MA, Kontarakis Z, Rossi A, Kuenne C, Günther S, Fukuda N, Kikhi K, Boezio GLM, Takacs CM, Lai SL, Fukuda R, Gerri C, Giraldez AJ, Stainier DYR (2019) Genetic compensation triggered by mutant mRNA degradation. Nature 568(7751):193–197.
Hagerman RJ, McBogg P, Hagerman PJ (1983) The fragile X syndrome: history, diagnosis, and treatment. J Dev Behav Pediatr 4:122–130
He CX, Portera-Cailliau C (2013) The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience 251:120–128
He H, Wang C, Tang Q, Yang F, Xu Y (2018) Possible mechanisms of prednisolone-induced osteoporosis in zebrafish larva. Biomed Pharmacother 101:981–987
Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M (1993) Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet 3:36–43
Hruscha A, Krawitz P, Rechenberg A, Heinrich V, Hecht J, Haass C, Schmid B (2013) Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 140:4982–4987
Ishii K, Nagaoka A, Kishida Y, Okazaki H, Yagishita S, Ucar H, Takahashi N, Saito N, Kasai H (2018) In vivo volume dynamics of dendritic spines in the neocortex of wild-type and Fmr1 KO mice. eNeuro 5
Kim L, He L, Maaswinkel H, Zhu L, Sirotkin H, Weng W (2014) Anxiety, hyperactivity and stereotypy in a zebrafish model of fragile X syndrome and autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry 55:40–49
Kjaer I, Hjalgrim H, Russell BG (2001) Cranial and hand skeleton in fragile X syndrome. Am J Med Genet 100:156–161
Levran O, Peles E, Randesi M, Correa da Rosa J, Ott J, Rotrosen J, Adelson M, Kreek MJ (2015) Synaptic plasticity and signal transduction gene polymorphisms and vulnerability to drug addictions in populations of European or African Ancestry. CNS Neurosci Ther 21(11):898–904
Liu B, Li Y, Stackpole EE, Novak A, Gao Y, Zhao Y, Zhao X, Richter JD (2018) Regulatory discrimination of mRNAs by FMRP controls mouse adult neural stem cell differentiation. Proc Natl Acad Sci USA 115:E11397–E11405
McNeil PL, Nebot C, Sloman KA (2016) Physiological and behavioral effects of exposure to environmentally relevant concentrations of prednisolone during zebrafish (Danio rerio) embryogenesis. Environ Sci Technol 50:5294–5304
Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, Rife M, Willemsen R, Nelson DL, Oostra BA (2006) The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis 21:549–555
Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST (2007) Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci USA 104:15537–15542
Ng MC, Yang YL, Lu KT (2013) Behavioral and synaptic circuit features in a zebrafish model of fragile X syndrome. PLoS ONE 8:e51456
O'Neale A, Ellis J, Creton R, Colwill RM (2014) Single stimulus learning in zebrafish larvae. Neurobiol Learn Mem 108:145–154
Remmers CL, Contractor A (2018) Development of GABAergic inputs is not altered in early maturation of adult born dentate granule neurons in fragile X Mice. eNeuro 5
Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC (2007) p53 activation by knockdown technologies. PLoS Genet 3:e78
Rossi A, Kontarakis Z, Gerri C, Nolte H, Hölper S, Krüger M, Stainier DY (2015) Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524(7564):230–233
Shamay-Ramot A, Khermesh K, Porath HT, Barak M, Pinto Y, Wachtel C, Zilberberg A, Lerer-Goldshtein T, Efroni S, Levanon EY, Appelbaum L (2015) Fmrp interacts with adar and regulates RNA editing, synaptic density and locomotor activity in zebrafish. PLoS Genet 11:e1005702
Tucker B, Richards R, Lardelli M (2004) Expression of three zebrafish orthologs of human FMR1-related genes and their phylogenetic relationships. Dev Genes Evol 214:567–574
Tucker B, Richards RI, Lardelli M (2006) Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum Mol Genet 15:3446–3458
Van’t Padje S, Engels B, Blonden L, Severijnen LA, Verheijen F, Oostra BA, Willemsen R (2005) Characterisation of Fmrp in zebrafish: evolutionary dynamics of the fmr1 gene. Dev Genes Evol 215:198–206
Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP et al (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914
Xu S, Elefant F (2015) Tip off the HAT—epigenetic control of learning and memory by Drosophila Tip60. Fly (Austin) 9(1):22–28
Yan J, Porch MW, Court-Vazquez B, Bennett MVL, Zukin RS (2018) Activation of autophagy rescues synaptic and cognitive deficits in fragile X mice. Proc Natl Acad Sci USA 115:E9707–E9716
Acknowledgements
This work was supported by grants from the Industry-University Collaboration Project of Jiangsu Province (BY2016049-02), National Natural Science Foundation of China (21876198), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Research Foundation of the Affiliated Children's Hospital of Xi'an Jiaotong University (2018A04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jia Hu, Lei Chen, Jian Yin, Huancai Yin, Yinong Huang, and Jingjing Tian declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures were approved by the Fudan University Animal Care and Use Committee and were performed in accordance with the governmental regulations of China.
Additional information
Handling Editor: Sonoko Ogawa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, J., Chen, L., Yin, J. et al. Hyperactivity, Memory Defects, and Craniofacial Abnormalities in Zebrafish fmr1 Mutant Larvae. Behav Genet 50, 152–160 (2020). https://doi.org/10.1007/s10519-020-09995-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-020-09995-7