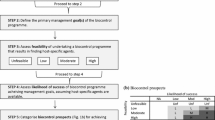
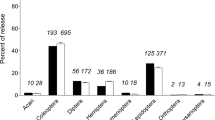
Abstract
A persistent problem in weed biocontrol is how to reliably predict whether a plant that supports development in laboratory host-specificity testing will be utilized in field conditions, and this is undoubtedly preventing releases of safe and effective agents. Moreover, the potential for unanticipated undesirable indirect effects of weed biocontrol on ecological networks has raised concerns by policy-makers and the general public. The key to minimizing risks of non-target impacts is prioritizing candidate agents that are both host-specific and effective, such that the number of agents required to bring the weed under control is minimized. As a consequence both the weed and its biocontrol agents become minor components of the local biota. Here we review recent attempts in New Zealand to improve the predictive ability of host-range testing, to avoid potentially safe and effective agents being rejected. Research in New Zealand aimed at predicting whether an agent is likely to experience enemy-release (i.e. reduced parasitism and predation) could assist agent prioritization, potentially making biocontrol both environmentally safer and more effective.
Similar content being viewed by others
References
Barton J (2012) Predictability of pathogen host range in classical biological control of weeds: an update. BioControl 57:289–305
Becker RL, Katovich EJS, Hinz HL, Gerber E, Ragsdale DW, Venette RC, McDougall DN, Reardon R, Riper LCv, Skinner LC, Landis DA (2013) The garlic mustard (Alliaria petiolata) case, what makes a good biological control target: the intersection of science, perspectives, policy and regulation. In: Proceedings of the XIII international symposium on biological control of weeds, Waikoloa, Hawaii, USA, 11-16 September, 2011, 2013, pp 332–339
Berry JA (2007) Alysiinae (Insecta: Hymenoptera: Braconidae). Fauna N Z 58:95
Carvalheiro LG, Buckley YM, Ventim R, Fowler SV, Memmott J (2008) Apparent competition can compromise the safety of highly specific biocontrol agents. Ecol Lett 11:690–700
Chen Y, Giles K, Payton M, Greenstone M (2000) Identifying key cereal aphid predators by molecular gut analysis. Mol Ecol 9:1887–1898
Coombs EM, Clark JK, Piper GL, Cofrancesco AF Jr (eds) (2004) Biological control of invasive plants in the United States. Oregon State University Press, Corvallis
Cristofaro M, De Biase A, Smith L (2013) Field release of a prospective biological control agent of weeds, Ceratapion basicorne, to evaluate potential risk to a nontarget crop. Biol Control 64:305–314
ERMA (2007) Environmental risk management authority decision NOR06005. http://www.epa.govt.nz/search-databases/HSNO%20Application%20Register%20Documents/NOR06005.doc. Accessed 11 May 2017
Fowler SV, Gourlay AH, Hill RH, Withers T (2004) Safety in New Zealand weed biocontrol: a retrospective analysis of host-specificity testing and the predictability of impacts on non-target plants. In: Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK (eds) Proceedings of the XI international symposium on biological control of weeds. CSIRO Entomology, Canberra, Australia, pp 265–270
Fowler SV, Paynter Q, Hayes L, Dodd S, Groenteman R (2010) Biocontrol of weeds in New Zealand: an overview of nearly 85 years. In: 17th Australasian weeds conference. New frontiers in New Zealand: together we can beat the weeds. Christchurch, New Zealand, 26–30 September, 2010, pp 211–214
Goeden RD, Louda SM (1976) Biotic interference with insects imported for weed control. Annu Rev Entomol 21:325–342
Groenteman R, Fowler SV, Sullivan JJ (2011) St. John’s wort beetles would not have been introduced to New Zealand now: a retrospective host range test of New Zealand’s most successful weed biocontrol agents. Biol Control 57:50–58
Hasan S, Delfosse ES (1995) Susceptibility of the Australian native, Heliotropium crispatum, to the rust fungus Uromyces heliotropii introduced to control common heliotrope, Heliotropium europaeum. Biocontrol Sci Technol 5:165–174
Heenan PB, de Lange PJ (2004) Alternanthera denticulata (amaranthaceae) in New Zealand: a new addition to the indigenous or naturalised flora? N Z J Bot 42:739–745
Hill R, Campbell D, Hayes L, Corin S, Fowler S (2013) Why the New Zealand regulatory system for introducing new biological control agents works. In: Wu Y, Johnson T, Sing S, Raghu S, Wheeler G, Pratt P, Warner K, Center T, Goolsby J, Reardon R (eds) Proceedings of the XIII international symposium on biological control of weeds. Morgantown, West Virginia, USA: USDA Forest Service Forest Health Technology Enterprise Team, pp 75–83
Hinz HL, Gassmann A, Bourchier RS, Schwarzlander M (2014) Successes we may not have had: a retrospective comparison of predicted versus realized host range of weed biological control agents in North America. Invasive Plant Sci Manag 7:564–579
Hosking J (1990) The feasibility of biological control of Cytisus scoparius (L.) Link. Report on overseas study tour, June-September 1990. Unpublished report, New South Wales Department of Agriculture and Fisheries, Tamworth, Australia
Julien MH, McFadyen R, Cullen J (2012) Biological control of weeds in Australia. CSIRO Publishing, Melbourne
Kirby M (2006) Population fluctuations in gorse mites. Trans Suffolk Nat Soc 42:55–61
Klein H (2011) A catalogue of the insects, mites and pathogens that have been used or rejected, or are under consideration, for the biological control of invasive alien plants in South Africa. Afr Entomol 19:515–549
Klein H, Hill M, Zachariades C, Zimmermann H (2011) Regulation and risk assessment for importations and releases of biological control agents against invasive alien plants in South Africa. Afr Entomol 19:488–497
Louda SM, Kendall D, Connor J, Simberloff D (1997) Ecological effects of an insect introduced for the biological control of weeds. Science 277:1088–1090
Louda SM, Pemberton RW, Johnson MT, Follett PA (2003) Nontarget effects - the Achilles’ heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annu Rev Entomol 48:365–396
Luck RF, Shepard BM, Kenmore P (1988) Experimental methods for evaluating arthropod natural enemies. Annu Rev Entomol 33:367–389
Mala VN (2013) The ecology and impact of the broom gall mite (Aceria genistae) on its host plant Scotch broom (Cytisus scoparius). M.Sc. Thesis. The University of Auckland
Manrique V, Diaz R, Condon T, Overholt W (2014) Host range tests reveal Paectes longiformis is not a suitable biological control agent for the invasive plant Schinus terebinthifolia. BioControl 59:761–770
Marohasy J (1998) The design and interpretation of host-specificity tests for weed biological control with particular reference to insect behaviour. Biocontrol News Inf 19:13N–20N
McFadyen R, Jacob HS (2004) Insects for the biocontrol of weeds: predicting parasitism levels in the new country. In: Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK (eds) Proceedings of the XI international symposium on biological control of weeds, 2004. CSIRO Entomology, Canberra, Australia, pp 135–140
Mortensen K (1984) A proposal for standardized scale of attack and its application to biocontrol agents of weeds in laboratory screening tests. In: Delfosse ES (ed) Proceedings of the VI international symposium on biological control of weeds. Agriculture Canada, pp 643–650
Paynter QE, Fowler SV, Gourlay AH, Haines ML, Harman HM, Hona SR, Peterson PG, Smith LA, Wilson-Davey JA, Winks CJ, Withers TM (2004) Safety in New Zealand weed biocontrol: a nationwide survey for impacts on non-target plants. N Z Plant Protect 57:102–107
Paynter Q, Gourlay AH, Oboyski PT, Fowler SV, Hill RL, Withers TM, Parish H, Hona S (2008a) Why did specificity testing fail to predict the field host-range of the gorse pod moth in New Zealand? Biol Control 46:453–462
Paynter Q, Martin N, Berry J, Hona S, Peterson P, Gourlay AH, Wilson-Davey J, Smith L, Winks C, Fowler SV (2008b) Non-target impacts of Phytomyza vitalbae a biological control agent of the European weed Clematis vitalba in New Zealand. Biol Control 44:248–258
Paynter Q, Fowler SV, Gourlay AH, Groenteman R, Peterson P, Smith L, Winks CJ (2010) Predicting parasitoid accumulation on biological control agents of weeds. J Appl Ecol 47:575–582
Paynter Q, Forgie SA, Winks CJ, Peterson PG, Ward DF, Nicholson L, van Zoelen R (2012) Biotic resistance: facilitation between invasive Homoptera and invasive ants limits the establishment of an introduced weed biocontrol agent in New Zealand. Biol Control 63:188–194
Paynter Q, Fowler SV, Hayes L, Hill RL (2015a) Factors affecting the cost of weed biocontrol programs in New Zealand. Biol Control 80:119–127
Paynter Q, Fowler SV, Hugh Gourlay A, Peterson PG, Smith LA, Winks CJ (2015b) Relative performance on test and target plants in laboratory tests predicts the risk of non-target attack in the field for arthropod weed biocontrol agents. Biol Control 80:133–142
Paynter Q, Fowler SV, Gourlay AH, Peterson PG, Smith LA, Winks CJ (2016) The influence of agent rearing success and release size on weed biocontrol programs in New Zealand. Biol Control 101:87–93
Paynter Q, Konuma A, Dodd SL, Hill RL, Field L, Gourlay AH, Winks CJ (2017) Prospects for biological control of Lonicera japonica (Caprifoliaceae) in New Zealand. Biol Control 105:56–65
Pearson DE, Callaway RM (2005) Indirect nontarget effects of host-specific biological control agents: implications for biological control. Biol Control 35:288–298
Peterson PG, McGregor PG, Springett BP (2000) Density dependent prey-feeding time of Stethorus bifidus (Coleoptera: Coccinellidae) on Tetranychus lintearius (Acari: Tetranychidae). N Z J Zool 27:41–44
Petráková L, Líznarová E, Pekár S, Haddad CR, Sentenská L, Symondson WOC (2015) Discovery of a monophagous true predator, a specialist termite-eating spider (Araneae: Ammoxenidae). Sci Rep 5:14013
Sheppard AW, Hill R, DeClerck-Floate RA, McClay A, Olckers T, Quimby PC, Zimmermann HG (2003) A global review of risk–benefit–cost analysis for the introduction of classical biological control agents against weeds: a crisis in the making? Biocontrol News Inf 24:77N–94N
Sheppard A, Haines M, Thomann T (2006) Native-range research assists risk analysis for non-targets in weed biological control: the cautionary tale of the broom seed beetle. Aust J Entomol 45:292–297
Simberloff D, Stiling P (1996) How risky is biological control? Ecology 77:1965–1974
Smith LA, Fowler SV, Paynter Q, Pedrosa-Macedo JH, Wigley P (2013) Successfully eliminating parasitic gregarines from Neolema ogloblini (Coleoptera: Chrysomelidae)—a biological control agent for Tradescantia fluminensis (Commelinaceae). In: Wu Y, Johnson T, Sing S, Raghu S, Wheeler G, Pratt P, Warner K, Center T, Goolsby J, Reardon R (eds) XIII international symposium on biological control of weeds. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team, 2012–2007, p 36
Strong DR (1997) Fear no weevil? Science 277:1058–1059
Suckling DM, Sforza RFH (2014) What magnitude are observed non-target impacts from weed biocontrol? PLoS ONE 9(1):e84847
van Driesche R, Bellows T, Elkinton J, Gould J, Ferro D (1991) The meaning of percentage parasitism revisited: solutions to the problem of accurately estimating total losses from parasitism. Environ Entomol 20:1–7
van Eyndhoven G (1967) Tetranychus lintearius Dufour, 1932, is a valid species (Acar.) Notalae ad Tetranychidas II. Entomol Ber 27:90–100
van Klinken RD (2000) Host specificity testing: why do we do it and how we can do it better. In: van Driesche R, Heard T, McClay A, Reardon R (eds) Host-specificity testing of exotic arthropod biological control agents: the biological basis for improvement in safety, USDA Forest Service Bulletin, Morgantown, West Virginia, USA, pp 54–68
van Klinken RD, Edwards OR (2002) Is host-specificity of weed biological control agents likely to evolve rapidly following establishment? Ecol Lett 5:590–596
Veldtman R, Lado TF, Botes A, Procheş Ş, Timm AE, Geertsema H, Chown SL (2011) Creating novel food webs on introduced Australian acacias: indirect effects of galling biological control agents. Divers Distrib 17:958–967
Wallis GP, Trewick SA (2009) New Zealand phylogeography: evolution on a small continent. Mol Ecol 18:3548–3580
Webb C, Sykes W, Garnock-Jones P (1988) Flora of New Zealand, vol IV. DSIR Botany Division, Christchurch
Willis AJ, Memmott J (2005) The potential for indirect effects between a weed, one of its biocontrol agents and native herbivores: a food web approach. Biol Control 35:299–306
Withers TM, Carlson CA, Gresham BA (2013) Statistical tools to interpret risks that arise from rare events in host specificity testing. Biol Control 64:177–185
Acknowledgements
This work was was supported by core funding to Landcare Research from the Ministry of Business, Innovation and Employment. QP thanks Mark Schwartzlaender for the opportunity to attend the International Congress of Entomology. We thank Cliff Moran, Mark Schwarzländer and S. Raghu and two anonymous reviewers for helpful comments on an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: S. Raghu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paynter, Q., Fowler, S.V. & Groenteman, R. Making weed biological control predictable, safer and more effective: perspectives from New Zealand. BioControl 63, 427–436 (2018). https://doi.org/10.1007/s10526-017-9837-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9837-5