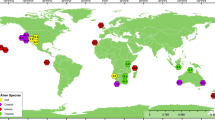
Abstract
The cosmopolitan reed grass Phragmites australis (Poaceae) is an intensively studied species globally with a substantial focus in the last two decades on its invasive populations. Here we argue that P. australis meets the criteria to serve as a model organism for studying plant invasions. First, as a dominant species in globally important wetland habitats, it has generated significant pre-existing research, demonstrating a high potential for funding. Second, this plant is easy to grow and use in experiments. Third, it grows abundantly in a wide range of ecological systems and plant communities, allowing a broad range of research questions to be addressed. We formalize the designation of P. australis as a model organism for plant invasions in order to encourage and standardize collaborative research on multiple spatial scales that will help to integrate studies on the ecology and evolution of P. australis invasive populations, their response to global environmental change, and implications for biological security. Such an integrative framework can serve as guidance for studying invasive plant species at the population level and global spatial scale.

Similar content being viewed by others
References
Able KW, Hagan SM, Brown SA (2003) Mechanisms of marsh habitat alteration due to Phragmites: response of young-of-the-year mummichog (Fundulus heteroclitus) to treatment for Phragmites removal. Estuaries 26:484–494
Achenbach L, Eller F, Nguyen LX, Brix H (2013) Differences in salinity tolerance of genetically distinct Phragmites australis clones. AoB Plants 5:plt019
Al-Garni SMS (2006) Increasing NaCl-salt tolerance of a halophytic plant Phragmites australis by mycorrhizal symbiosis. Am Eurasian J Agric Environ Sci 1:119–126
Allen WJ, Young RE, Bhattarai G, Croy JR, Meyerson LA, Cronin JT (2015) Enemy release and invasional meltdown of introduced plants and herbivores: Phragmites australis and Lipara spp. in North America. Biol Invasions 17:3419–3432
Bastlová D, Bastl M, Čižková H, Květ J (2006) Plasticity of Lythrum salicaria and Phragmites australis growth characteristics across a European geographical gradient. Hydrobiologia 570:237–242
Bhattarai GP, Cronin JT (2014) Hurricane activity and the large-scale pattern of spread of an invasive plant species. PLoS One 9:e98478. doi:10.1371/journal.pone.0098478
Bhattarai GP, Meyerson LA, Anderson J, Cummings D, Allen WJ, Cronin J (in review) The biogeography of a plant invasion: genetic variation and plasticity in latitudinal clines for plant-herbivore interaction traits. Ecol Monogr
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889
Bossdorf O, Richards CL, Pigliucci M (2008) Epigenetics for ecologists. Ecol Lett 11:106–115
Brix H (1994) Use of constructed wetlands in water pollution control: historical development, present status, and future perspectives. Water Sci Technol 30:209–224
Brix H (1999) The European research project on reed die-back and progression (EUREED). Limnologica 29:5–10
Broennimann O, Treier UA, Muller-Schärer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709
Canavan S, Wilson JRU, Visser V, Le Roux JJ, Vorontsova MS, Richardson DM (2016) The global dissemination of bamboos (Poaceae: Bambusoideae): a review. AoB plants
Caplan JS, Wheaton CN, Mozdzer TJ (2014) Belowground advantages in construction cost facilitate a cryptic plant invasion. AoB Plants. doi:10.1093/aobpla/plu020
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of reed into tidal wetlands of North America. Aquat Bot 64:261–273
Čížková H, Strand JA, Lukavská J (1996) Factors associated with reed decline in a eutrophic fishpond, Rožmberk (South Bohemia, Czech Republic). Folia Geobot Phytotaxon 31:73–84
Clay K, Shearin ZRC, Bourke KA, Bickford WA, Kowalski KP (2016) Diversity of fungal endophytes in non-native Phragmites australis in the Great Lakes. Biol Invasions. doi:10.1007/s10530-016-1137-y
Clevering OA, Lissner J (1999) Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis. Aquat Bot 64:185–208
Colautti RI, Franks SJ, Hufbauer RA, Kotanen P, Torchin M, Byers JE, Pyšek P, Bossdorf O (2014) The Global Garlic Mustard Field Survey: challenges and opportunities of a unique, large-scale collaboration for invasion biology. NeoBiota 21:29–47
Crocker EV, Karp MA, Nelson EB (2015) Virulence of oomycete pathogens from Phragmites australis-invaded and noninvaded soils to seedlings of wetland plant species. Ecol Evol 5:2127–2139
Cronin JT, Bhattarai G, Allen WJ, Meyerson LA (2015) Biogeography of a plant invasion: plant-herbivore interactions. Ecology 96:1115–1127
Cronin JT, Kiviat E, Meyerson LA, Bhattarai GP, Allen WJ (2016) Biological control of invasive Phragmites will be detrimental to native Phragmites. Biol Invasions. doi:10.1007/s10530-016-1138-x
Dibble KL, Meyerson LA (2012) Tidal flushing restores the physiological condition of fish residing in degraded salt marshes. PLoS One. doi:10.1371/journal.pone.0046161
Dibble KL, Meyerson LA (2013) The effects of plant invasion and ecosystem restoration on energy flow through salt marsh food webs. Estuaries Coasts. doi:10.1007/s12237-013-9673-5
Dibble KL, Meyerson LA (2016) Detection of decreased quantities of spawning capable Fundulus heteroclitus in tidally restricted marshes relative to restored and reference sites. Biol Invasions (in review)
Dibble KL, Pooler P, Meyerson LA (2013) Impacts of plant invasions can be reversed through restoration: a regional meta-analysis of faunal communities. Biol Invasions 15:1725–1737
Douhovnikoff V, Spens A (2016) Epigenetic variation within Phragmites australis among lineages, genotypes, and ramets. Biol Invasions (in review)
Dykyjová D, Hejný S, Květ J (1973) Proposal for international comparative investigations of production by stands of reed (Phragmites communis). Folia Geobot Phytotaxon 8:435–442
Egan D (2001) A new acid test for ecological restoration. Ecol Restor 19:4
Eller F, Lambertini C, Nguyen LX, Brix H (2014) Increased invasive potential of non-native Phragmites australis: elevated CO2 and temperature alleviate salinity effects on photosynthesis and growth. Glob Change Biol 20:531–543
Farnsworth EJ, Meyerson LA (1999) Species composition and inter-annual dynamics of a freshwater tidal plant community following removal of the invasive grass, Phragmites australis. Biol Invasions 1:115–127
Gilmore MS, Wilson EH, Barrett N, Civco DL, Prisloe S, Hurd JD, Chadwick C (2008) Integrating multi-temporal spectral and structural information to map wetland vegetation in a lower Connecticut River tidal marsh. Remote Sens Environ 112:4048–4060
Gratton C, Denno RF (2006) Arthropod food web restoration following removal of an invasive wetland plant. Ecol Appl 16:622–631
Guo W, Lambertini C, Li X-Z, Meyerson LA, Brix H (2013) Invasion of Old World Phragmites australis in the New World: precipitation and human influences redesign the invasive niche. Glob Change Biol 19:3406–3422
Harley JL, Harley EL (1987) A checklist of mycorrhiza in the British flora–addenda, errata and index. New Phytol 107:741–749
Haslam S (1971a) Community regulation in Phragmites communis Trin. I. Mono dominant stands. J Ecol 59:65–73
Haslam S (1971b) Community regulation in Phragmites communis Trin. II. Mixed stands. J Ecol 59:75–88
Hazelton ELG, Mozdzer TJ, Burdick DM, Kettenring KM, Whigham DF (2014) Phragmites australis management in the United States: 40 years of methods and outcomes. AoB Plants 6:plu001
Holdredge C, Bertness MD, von Wettberg E, Silliman BR (2010) Nutrient enrichment enhances hidden differences in phenotype to drive a cryptic plant invasion. Oikos 119:1776–1784
Holt RD, Lawton JH (1994) The ecological consequences of shared natural enemies. Annu Rev Ecol Syst 25:495–520
Hughes AR, Schenck FR, Bloomberg J, Hanley TC, Tarik DF, Gouhier C, Beighley RE, Kimbro DL (2016) Biogeographic gradients in ecosystem processes of the invasive ecosystem engineer Phragmites australis. Biol Invasions (in revision)
Hulme P, Pyšek P, Jarošik V, Pergl J, Schaffner U, Vila M (2013) Bias and error in understanding invasions and impacts. Trends Ecol Evol 28:212–218
Hunter KL, Fox DA, Brown LM, Able KW (2006) Responses of resident marsh fishes to stages of Phragmites australis invasion in three mid Atlantic estuaries. Estuaries Coasts 29:487–498
Isac M, Preda S, Marcu M (1998) Aphid species–vectors of plum pox virus. Acta Virol 42:233–234
Kiviat E (2013) Ecosystem services of Phragmites in North America with emphasis on habitat functions. AoB plants 5:plt008
Kowalski KP, Bacon C, Bickford W, Braun H, Clay K, Leduc-Lapierre M, Lillard E, McCormick MK, Nelson E, Torres M, White J, Wilcox DA (2015) Advancing the science of microbial symbiosis to support invasive species management: a case study on Phragmites in the Great Lakes. Front Microbiol 6:95. doi:10.3389/fmicb.2015.00095
Kueffer C, Pyšek P, Richardson DM (2013) Integrative invasion science: model systems, multi-site studies, focused meta-analysis, and invasion syndromes. New Phytol 200:615–633
Lambert A, Dudley T, Saltonstall K (2010) Ecology and Impacts of the large-statured invasive grasses Arundo donax and Phragmites australis in North America. Invasive Plant Sci Manag 3:489–494
Lambert A, Saltonstall K, Long R, Dudley T (2016) Biogeography of Phragmites lineages in the southwestern United States. Biol Invasions (in review)
Lambertini C, Gustafsson MHG, Frydenberg J, Lissner J, Speranza M, Brix H (2006) A phylogeographic study of the cosmopolitan genus Phragmites (Poaceae) based on AFLPs. Plant Syst Evol 258:161–182
Lambertini C, Mendelssohn IA, Gustafsson MHG, Olese B, Riis T, Sorrell BK, Brix H (2012) Tracing the origin of Gulf Coast Phragmites (Poaceae): a story of long-distance dispersal and hybridization. Am J Bot 99:538–551
Lozier JD, Roderick GK, Mills NJ (2009) Tracing the invasion history of mealy plum aphid, Hyalopterus pruni (Hemiptera: Aphididae), in North America: a population genetics approach. Biol Invasions 11:299–314
Marks M, Lapin B, Randall J (1994) Phragmites australis (P. communis): threats, management and monitoring. Nat Areas J 14(4):285–294
Martin L, Blossey B (2013) The runaway weed: costs and failures of Phragmites australis management in the USA. Estuaries Coasts 36:626–632
Meyerson LA, Cronin JT (2013) Evidence for multiple introductions of Phragmites australis to North America: detection of a new non-native haplotype. Biol Invasions 15:2605–2608
Meyerson LA, Chambers RM, Vogt KA (1999) The effects of Phragmites removal on nutrient pools in a freshwater tidal marsh ecosystem. Biol Invasions 1:129–136
Meyerson LA, Vogt KA, Chambers RM (2000a) Linking the success of Phragmites to the alteration of ecosystem nutrient cycles. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Springer, Netherlands, pp 827–844
Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S (2000b) A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecol Manag 8:89–103
Meyerson LA, Saltonstall K, Chambers RM (2009) Phragmites australis in eastern North America: a historical and ecological perspective. In: Silliman BR, Grosholz ED, Bertness MD (eds) Human impacts on salt marshes: a global perspective. University of California Press, Berkeley, pp 57–82
Meyerson LA, Lambert AM, Saltonstall K (2010a) A tale of three lineages: expansion of common reed (Phragmites australis) in the U.S. Southwest and Gulf Coast. Invasive Plant Sci Manag 3:515–520
Meyerson LA, Viola DV, Brown RN (2010b) Hybridization of invasive Phragmites australis with a native subspecies in North America. Biol Invasions 12:103–111
Meyerson LA, Lambertini C, McCormick MK, Whigham DF (2012) Hybridization of common reed in North America? The answer is blowing in the wind. AoB plants 2012:pls022
Meyerson LA, Pergl J, Pyšek P (2014) Making waves about spreading weeds. Science 344:1236
Meyerson LA, Cronin JT, Bhattarai GP, Brix H, Lambertini C, Lučanová M, Rinehart S, Suda J, Pyšek P (2016) Ploidy level and nuclear genome size modify the expression of plant traits and response to herbivory in Phragmites australis. Biol Invasions (in revision)
Mozdzer TJ, Megonigal JP (2013) Increased methane emissions by an introduced Phragmites australis lineage under global change. Wetlands 33:609–615
Mozdzer TJ, Brisson J, Hazelton E (2013) Physiological ecology and functional traits of North American native and Eurasian introduced Phragmites australis lineages. AoB Plants 5:plt048
Nelson EB, Karp MA (2013) Soil pathogen communities associated with native and non-native Phragmites australis populations in freshwater wetlands. Ecol Evol 3:5254–5267
Oliveira RS, Dodd JC, Castro PML (2001) The mycorrhizal status of Phragmites australis in several polluted soils and sediments of an industrialised region of Northern Portugal. Mycorrhiza 10:241–247
Packer J, Meyerson LA, Skálová H, Haslam S, Pyšek P, Kueffer C (2016) Biological Flora of the British Isles: Phragmites australis. J Ecol (in review)
Parker JD, Torchin ME, Hufbauer RA, Lemoine NP, Alba C, Blumenthal DM, Bossdorf O, Byers JE, Dunn AM, Heckman RW, Hejda M, Jarošík V, Kanarek AR, Martin LB, Perkins SE, Pyšek P, Schierenbeck K, Schlöder C, van Klinken R, Vaughn KJ, Williams W, Wolfe LM (2013) Do invasive species perform better in their new ranges? Ecology 94:985–994
Pellegrin D, Hauber DP (1999) Isozyme variation among populations of the clonal species, Phragmites australis (Cav.) Trin. ex Steudel. Aquat Bot 63:241–259
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ (2013) Adaptive evolution in invasive species. Trends Plant Sci 13:288–294
Pyšek P, Richardson DM, Pergl J, Jarošík V, Sixtová Z, Weber E (2008) Geographical and taxonomic biases in invasion ecology. Trends Ecol Evol 23:237–244
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci 99:2445–2449
Saltonstall K (2003a) Microsatellite variation within and among North American lineages of Phragmites australis. Mol Ecol 12:1689–1702
Saltonstall K (2003b) A rapid method for identifying the origin of North American Phragmites populations using RFLP analysis. Wetlands 23:1043–1047
Saltonstall K, Peterson PM, Soreng RJ (2004) Recognition of Phragmites australis subsp. americanus (Poaceae: Arundinoideae) in North America: evidence from morphological and genetic analyses. SIDA 21:683–692
Saltonstall K, Glennon K, Burnett A, Hunter RB, Hunter KL (2007) Comparison of morphological variation indicative of ploidy level in Phragmites australis (Poaceae) from eastern North America. Rhodora 109:415–429
Saltonstall K, Castillo H, Blossey B (2014) Confirmed field hybridization of native and introduced Phragmites australis (Poaceae) in North America. Am J Bot 101:211–215
Saltonstall K, Lambert A, Rice N (2016) What happens in Vegas, better stay in Vegas: Phragmites australis hybrids in the Las Vegas Wash. Biol Invasions (in revision)
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Soares MA, Li H-Y, Kowalski KP, Bergen M, Torres MS, White JF (2016) Evaluation of the functional roles of fungal endophytes of Phragmites australis from high saline and low saline habitats. Biol Invasions (this issue)
Suda J, Meyerson LA, Pyšek P, Leitch I (2014) The hidden side of plant invasions: the role of genome size. New Phytol 205:994–1007
Swearingen J, Saltonstall K (2010) Phragmites field guide: distinguishing native and exotic forms of common reed (Phragmites australis) in the United States. Weeds Gone Wild, Plant Conservation Alliance (http://www.nps.gov/plants/alien/fact/pdf/phau1-powerpoint.pdf)
Tscharntke T (1992) Cascade effects among four trophic levels: bird predation on galls affects density-dependent parasitism. Ecology 73:1689–1698
Weber E (2003) Invasive plant species of the world: a reference guide to environmental weeds. CAB International Publishing, Wallingford
Wu J, Ma F, Wang L, Yang J, Huang X, An G, Liu S (2014) Seedling performance of Phragmites australis (Cav.) Trin ex. Steudel in the presence of arbuscular mycorrhizal fungi. J Appl Microbiol 116:1593–1606
Wu CA, Murray LA, Heffernan KE (2015) Evidence for natural hybridization between native and introduced lineages of Phragmites australis (Poaceae) in the Chesapeake Bay watershed. Am J Bot 102:805–812
Yarwood S, Baldwin A, Mateu MG, Buyer J (2016) Archaeal rhizosphere communities differ between the native and invasive lineages of the wetland plant Phragmites australis (common reed) in a Chesapeake Bay subestuary. Biol Invasions. doi:10.1007/s10530-016-1144-z
Zhang Q, Sun Q, Koide RT, Peng Z, Zhou J, Gu X, Gao W, Yu M (2014) Arbuscular mycorrhizal fungal mediation of plant–plant interactions in a marshland plant community. Sci World J. doi:10.1155/2014/923610
Acknowledgments
LAM and JTC were supported by NSF research Grant 1049914 and 1050084. PP and LAM were supported by the Czech Science Foundation (Project No. 14-15414S), PP also by long-term research development project RVO 67985939 (The Czech Academy of Sciences) and Praemium Academiae award from The Czech Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Laura A. Meyerson and Kristin Saltonstall/Phragmites invasion.
Rights and permissions
About this article
Cite this article
Meyerson, L.A., Cronin, J.T. & Pyšek, P. Phragmites australis as a model organism for studying plant invasions. Biol Invasions 18, 2421–2431 (2016). https://doi.org/10.1007/s10530-016-1132-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1132-3