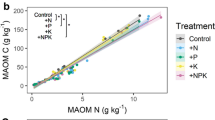
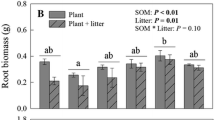
Abstract
Humans have dramatically increased the deposition and availability of nutrients, such as nitrogen (N), worldwide. Soil organic matter (SOM) is a significant global reservoir of carbon (C); however, the effects of N enrichment on this large, heterogeneous C stock are unclear. Nitrogen has variable effects on the biological, chemical, and physical factors that determine SOM pool mean residence time; consequently, we predicted that N enrichment would have distinct effects on SOM pools, including the pool that is readily available for microbial decomposition, as well as the pools that have been stabilized against microbial decomposition via aggregate occlusion and mineral association. We addressed this gap in knowledge by measuring the effects of N addition on different SOM pools at five grassland experiments in the US Central Great Plains that participate in the Nutrient Network and have been fertilized for three or five years. Overall, N addition decreased microbial respiration of unoccluded OM by as much as 29 % relative to control plots, and consequently, decreased C loss from this pool. Furthermore, N addition tended to increase soil aggregation and C occlusion in large macro-aggregates. These results suggest that N addition will increase C sequestration by slowing the decomposition of SOM, as well as stabilizing SOM against microbial decomposition in aggregate-occluded pools. However, the effects of N on all pools studied varied among sites, possibly due to site variation in soil texture. Consequently, increased sequestration of soil C in response to N enrichment may not be universal across grasslands.


Similar content being viewed by others
References
Ågren GI, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–98. doi:10.1007/s004420100646
Ashworth J, Keyes D, Kirk R, Lessard R (2001) Standard procedure in the hydrometer method for particle size analysis. Commun Soil Sci Plant Anal 32:633–642. doi:10.1081/CSS-100103897
Bach EM, Baer SG, Meyer CK, Six J (2010) Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol Biochem 42:2182–2191. doi:10.1016/j.soilbio.2010.08.014
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31:697–710
Beck T, Joergensen RG, Kandeler E et al (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29:1023–1032. doi:10.1016/S0038-0717(97)00030-8
Berg B, Matzner E (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25. doi:10.1139/a96-017
Berg B, Staaf H (1980) Decomposition rate and chemical changes of Scots pine needle litter. II. Influence of chemical composition. Ecol Bull 32:373–390
Beven K (2006) A manifesto for the equifinality thesis. J Hydrol 320:18–36. doi:10.1016/j.jhydrol.2005.07.007
Borer ET, Harpole WS, Adler PB et al (2014) Finding generality in ecology: a model for globally distributed experiments. Methods Ecol Evol 5:65–73. doi:10.1111/2041-210X.12125
Bouwman A, Van Vuuren D, Derwent R, Posch M (2002) A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut 141:349–382. doi:10.1023/A:1021398008726
Bradford MA, Fierer N, Jackson RB et al (2008) Nonlinear root-derived carbon sequestration across a gradient of nitrogen and phosphorous deposition in experimental mesocosms. Glob Change Biol 14:1113–1124. doi:10.1111/j.1365-2486.2008.01564.x
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. doi:10.1016/0038-0717(85)90144-0
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Ciais P, Sabine C, Bala G et al (2013) Carbon and other biogeochemical cycles. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Cornwell WK, Cornelissen JH, Amatangelo K et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cotrufo MF, Wallenstein MD, Boot CM et al (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995. doi:10.1111/gcb.12113
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113. doi:10.1890/06-1847.1
Dentener FJ (2006) Global maps of atmospheric nitrogen deposition, 1860, 1993, and 2050. Data set. Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge. http://daac.ornl.gov/
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol 18:1781–1796. doi:10.1111/j.1365-2486.2012.02665.x
Elliott ET, Palm CA, Reuss DE, Monz CA (1991) Organic matter contained in soil aggregates from a tropical chronosequence: correction for sand and light fraction. Agric Ecosyst Environ 34:443–451. doi:10.1016/0167-8809(91)90127-J
Feng X, Simpson AJ, Schlesinger WH, Simpson MJ (2010) Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest. Glob Change Biol 16:2104–2116. doi:10.1111/j.1365-2486.2009.02080.x
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Fornara DA, Tilman D (2012) Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology 93:2030–2036
Frey SD, Ollinger S, Nadelhoffer K et al (2014) Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121:305–316. doi:10.1007/s10533-014-0004-0
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. doi:10.1126/science.1136674
Gillespie AW, Diochon A, Ma BL et al (2014) Nitrogen input quality changes the biochemical composition of soil organic matter stabilized in the fine fraction: a long-term study. Biogeochemistry 117:337–350. doi:10.1007/s10533-013-9871-z
Gough L, Osenberg CW, Gross KL, Collins SL (2000) Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89:428–439. doi:10.1034/j.1600-0706.2000.890302.x
Guggenberger G, Frey SD, Six J et al (1999) Bacterial and fungal cell-wall residues in conventional and no-tillage agroecosystems. Soil Sci Soc Am J 63:1188. doi:10.2136/sssaj1999.6351188x
Gupta VVSR, Germida JJ (2015) Soil aggregation: influence on microbial biomass and implications for biological processes. Soil Biol Biochem 80:A3–A9. doi:10.1016/j.soilbio.2014.09.002
Hagedorn F, Spinnler D, Siegwolf R (2003) Increased N deposition retards mineralization of old soil organic matter. Soil Biol Biochem 35:1683–1692. doi:10.1016/j.soilbio.2003.08.015
Henry HAL, Cleland EE, Field CB, Vitousek PM (2004) Interactive effects of elevated CO2, N deposition and climate change on plant litter quality in a California annual grassland. Oecologia 142:465–473. doi:10.1007/s00442-004-1713-1
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Hobbie SE, Eddy WC, Buyarski CR et al (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr. doi:10.1890/11-1600.1
Hood-Nowotny R, Umana NH-N, Inselbacher E et al (2010) Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Sci Soc Am J 74:1018–1027
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322. doi:10.1038/ngeo844
Jastrow JD, Miller RM, Boutton TW (1996) Carbon dynamics of aggregate-associated organic matter estimated by carbon-13 natural abundance. Soil Sci Soc Am J 60:801–807. doi:10.2136/sssaj1996.03615995006000030017x
Jastrow J, Miller R, Lussenhop J (1998) Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol Biochem 30:905–916
Kaye J, Barrett J, Burke I (2002) Stable nitrogen and carbon pools in grassland soils of variable texture and carbon content. Ecosystems 5:461–471. doi:10.1007/s10021-002-0142-4
Keeler BL, Hobbie SE, Kellogg LE (2008) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15. doi:10.1007/s10021-008-9199-z
King GM (2011) Enhancing soil carbon storage for carbon remediation: potential contributions and constraints by microbes. Trends Microbiol 19:75–84. doi:10.1016/j.tim.2010.11.006
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379. doi:10.1890/06-2057.1
Lee M, Manning P, Rist J et al (2010) A global comparison of grassland biomass responses to CO2 and nitrogen enrichment. Philos Trans R Soc B 365:2047–2056
Li W, Jin C, Guan D et al (2015) The effects of simulated nitrogen deposition on plant root traits: a meta-analysis. Soil Biol Biochem 82:112–118. doi:10.1016/j.soilbio.2015.01.001
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828. doi:10.1111/j.1461-0248.2010.01482.x
Lu M, Zhou X, Luo Y et al (2011) Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agric Ecosyst Environ 140:234–244. doi:10.1016/j.agee.2010.12.010
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Mueller KE, Eissenstat DM, Hobbie SE et al (2012) Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 111:601–614. doi:10.1007/s10533-011-9695-7
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Neff JC, Townsend AR, Gleixner G et al (2002) Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 419:915–917
Oades JM (1984) Soil organic matter and structural stability: mechanisms and implications for management. Plant Soil 76:319–337
Oades JM (1988) The retention of organic matter in soils. Biogeochemistry 5:35–70
Oades J, Waters A (1991) Aggregate hierarchy in soils. Soil Res 29:815–828
Plante AF, McGill WB (2002) Soil aggregate dynamics and the retention of organic matter in laboratory-incubated soil with differing simulated tillage frequencies. Soil Tillage Res 66:79–92
Reid J, Adair E, Hobbie S, Reich P (2012) Biodiversity, nitrogen deposition, and CO2 affect grassland soil carbon cycling but not storage. Ecosystems 15:580–590. doi:10.1007/s10021-012-9532-4
Rillig MC (2004) Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci 84:355–363. doi:10.4141/S04-003
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol. doi:10.3389/fmicb.2012.00348
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404. doi:10.1016/j.soilbio.2009.10.014
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311. doi:10.1016/0038-0717(94)90211-9
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Six J, Paustian K, Elliott ET, Combrink C (2000) Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci Soc Am J 64:681–689
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res 79:7–31
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555. doi:10.2136/sssaj2004.0347
Sollins P, Swanston C, Kleber M et al (2006) Organic C and N stabilization in a forest soil: evidence from sequential density fractionation. Soil Biol Biochem 38:3313–3324
Spohn M (2015) Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 12:817–823. doi:10.5194/bg-12-817-2015
Strickland MS, Rousk J (2010) Considering fungal:bacterial dominance in soils—methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395. doi:10.1016/j.soilbio.2010.05.007
Talbot JM, Treseder KK (2011) Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93:345–354. doi:10.1890/11-0843.1
Thiet RK, Frey SD, Six J (2006) Do growth yield efficiencies differ between soil microbial communities differing in fungal:bacterial ratios? Reality check and methodological issues. Soil Biol Biochem 38:837–844. doi:10.1016/j.soilbio.2005.07.010
Torn MS, Trumbore SE, Chadwick OA et al (1997) Mineral control of soil organic carbon storage and turnover. Nature 389:170–173. doi:10.1038/38260
Torn MS, Vitousek PM, Trumbore SE (2005) The influence of nutrient availability on soil organic matter turnover estimated by incubations and radiocarbon modeling. Ecosystems 8:352–372. doi:10.1007/s10021-004-0259-8
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Von Lützow M, Kögel-Knabner I, Ekschmitt K et al (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions–a review. Eur J Soil Sci 57:426–445
Waldrop MP, Zak DR, Sinsabaugh RL et al (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–1177
Watson RT, Noble IR, Bolin B et al (2000) Land use, land-use change, and forestry. Special Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Wedin DA, Tilman D (1996) Influence of nitrogen loading and species composition on the carbon balance of grasslands. Science 274:1720
Wiles LJ, Barlin DH, Schweizer EE, et al (1996) A new soil sampler and elutriator for collecting and extracting weed seeds from soil. Weed Technol 10:35–41
Wilson GW, Rice CW, Rillig MC et al (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12:452–461
Yang X-M, Wander MM (1998) Temporal changes in dry aggregate size and stability: tillage and crop effects on a silty loam Mollisol in Illinois. Soil Tillage Res 49:173–183
Zak DR, Holmes WE, Burton AJ et al (2008) Simulated atmospheric NO3-deposition increases soil organic matter by slowing decomposition. Ecol Appl 18:2016–2027
Zeglin LH, Stursova M, Sinsabaugh RL, Collins SL (2007) Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154:349–359. doi:10.1007/s00442-007-0836-6
Acknowledgments
We thank Nutrient Network collaborators for establishing and maintaining the sites included in this study, including Elizabeth Borer, Adam Kay, and Eric Seabloom at Cedar Creek; Jean Knops at Cedar Point; Lori Biederman, Stan Harpole, Lauren Sullivan, and Ryan Williams at Chichaqua Bottoms; Kim LaPierre at Konza; and Dana Blumenthal, Cynthia Brown, and Julia Klein at Shortgrass Steppe. Numerous individuals at the University of Minnesota and Iowa State University assisted us in the lab and field, including: Daniel Ackerman, Rick Beckel, Chris Buyarski, Katie Kemmitt, Joey Krenz, Eric Lind, Elyssa McFarland, Queenster Nartey, Jennifer Pederson, Kristen Peterson, Missy Rudeen, Kelsey Thurow, and Michael Wells. Eric Lind also provided valuable assistance with data analysis and Nutrient Network database management. This work was supported by the Cedar Creek Long Term Ecological Research Program (NSF DEB-1234162), a National Science Foundation Graduate Research Fellowship to CER (Grant No. 00039202), and the Dayton Fund of the Bell Museum of Natural History. The Nutrient Network has been supported by funding to Elizabeth Borer and Eric Seabloom from the National Science Foundation Research Coordination Network and the Long Term Ecological Research programs, as well as the University of Minnesota’s Institute on the Environment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: E. Matzner.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riggs, C.E., Hobbie, S.E., Bach, E.M. et al. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 125, 203–219 (2015). https://doi.org/10.1007/s10533-015-0123-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0123-2