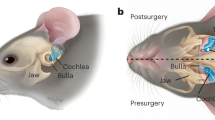
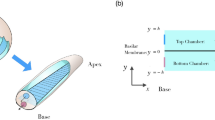
Abstract
One of the major challenges in treatment of auditory disorders is that many therapeutic compounds are toxic when delivered systemically. Local intracochlear delivery methods are becoming critical in emerging treatments and in drug discovery. Direct infusion via cochleostomy, in particular, is attractive from a pharmacokinetics standpoint, as there is potential for the kinetics of delivery to be well-controlled. Direct infusion is compatible with a large number of drug types, including large, complex molecules such as proteins and unstable molecules such as siRNA. In addition, hair-cell regeneration therapy will likely require long-term delivery of a timed series of agents. This presents unknown risks associated with increasing the volume of fluid within the cochlea and mechanical damage caused during delivery. There are three key requirements for an intracochlear drug delivery system: (1) a high degree of miniaturization (2) a method for pumping precise and small volumes of fluid into the cochlea in a highly controlled manner, and (3) a method for removing excess fluid from the limited cochlear fluid space. To that end, our group is developing a head-mounted microfluidics-based system for long-term intracochlear drug delivery. We utilize guinea pig animal models for development and demonstration of the device. Central to the system is an infuse-withdraw micropump component that, unlike previous micropump-based systems, has fully integrated drug and fluid storage compartments. Here we characterize the infuse-withdraw capabilities of our micropump, and show experimental results that demonstrate direct drug infusion via cochleostomy in animal models. We utilized DNQX, a glutamate receptor antagonist that suppresses CAPs, as a test drug. We monitored the frequency-dependent changes in auditory nerve CAPs during drug infusion, and observed CAP suppression consistent with the expected drug transport path based on the geometry and tonotopic organization of the cochlea.
















Similar content being viewed by others
References
Eaton-Peabody Laboratories (2014). http://www.masseyeandear.org/research/otolaryngology/investigators/laboratories/eaton-peabody-laboratories/epl-engineering-resources/epl-cochlear-function-test-suite/
J. Andrews, A. Böhmer, L. Hoffman, The measurement and manipulation of intralabyrinthine pressure in experimental endolymphatic hydrops. Laryngoscope. 101, 661–668 (1991)
S.E. Barron, R.P. Bobbin, P. Guth, C. Norris, Cytochalasin D suppresses sound evoked potentials in the guinea pig cochlea. Hear Res. 31(2), 147–53 (1987)
L. Bianchi, Y. Raz, Methods for providing therapeutic agents to treat damaged spiral ganglion neurons. Curr. Drug Targets-CNS Neurol. Disord. 3, 195–199 (2004)
R.P. Bobbin, G. Ceasar, Kynurenic acid and γ-D-glutamylaminomethylsulfonic the compound action potential of the auditory acid suppress nerve. Hear Res. 25, 77–81 (1987)
A. Böhmer, On the pathomechanism of cochlear dysfunction in experimental perilymph fistulas. Laryngoscope. 101, 1307–1312 (1991)
A. Böhmer, J. Andrews, Maintenance of hydrostatic pressure gradients in the membranous labyrinth. Arch Otorhinolaryngol. 246, 65–66 (1989)
S.S. Chandrasekhar, R.Y. Rubinstein, Kwartler Ja, M. Gatz, P.E. Connelly, E. Huang, S. Baredes, Dexamethasone pharmacokinetics in the inner ear: Comparison of route of administration and use of facilitating agents. Otolaryngol – Head Neck Surg. 122(4), 521–528 (2000). doi:10.1067/mhn.2000.102578
Z. Chen, S.G. Kujawa, M.J. McKenna, J.O. Fiering, M.J. Mescher, J.T. Borenstein, E.E.L. Swan, W.F. Sewell, Inner ear drug delivery via a reciprocating perfusion system in the guinea pig. J. Control. Rel. 110(1), 1–19 (2005). doi:10.1016/j.jconrel.2005.09.003
Z. Chen, A.A. Mikulec, M.J. McKenna, W.F. Sewell, S.G. Kujawa, A method for intracochlear drug delivery in the mouse. J. Neurosci. Methods. 150(1), 67–73 (2006). doi:10.1016/j.jneumeth.2005.05.017
G.P. Chrousos, in Adrenocorticosteroids and Adrenocortical Antagonists, ed. by B. Katzung. Adrenocorticosteroids Adrenocortical Antagon., (McGraw-Hill New York, 2007), pp. 635–652
K.J. Doyle, C. Bauch, R. Battista, C. Beatty, G.B. Hughes, J. Mason, J. Maw, F.L. Musiek, Intratympanic steroid treatment: a review. Otol. Neurotol. 25(6), 1034–9 (2004)
J. Eggermont, Tinnitus: neurobiological substrates. Drug Discov. Today. 10(19), 1283–1290 (2005)
T. Endo, T. Nakagawa, T. Kita, F. Iguchi, T.S. Kim, T. Tamura, K. Iwai, Y. Tabata, J. Ito, Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 115(11), 2016–20 (2005). doi:10.1097/01.mlg.0000183020.32435.59
J. Fiering, M.J. Mescher, Swan E.E. Leary, M.E. Holmboe, Ba. Murphy, Z. Chen, M. Peppi, W.F. Sewell, M.J. McKenna, S.G. Kujawa, J.T. Borenstein, Local drug delivery with a self-contained, programmable, microfluidic system. Biomed. Microdevice. 11(3), 571–8 (2009). doi:10.1007/s10544-008-9265-5
A. Fridberger, J.T. van Maarseveen, E. Scarfone, M. Ulfendahl, B. Flock, A. Flock, Pressure-induced basilar membrane position shifts and the stimulus-evoked potentials in the low-frequency region of the guinea pig cochlea. Acta Physiol. Scand. 161(2), 239–52 (1997). doi:10.1046/j.1365-201X.1997.00214.x
J.R. García-Berrocal, R. Ramírez-Camacho, D. Lobo, A. Trinidad, J.M. Verdaguer, Adverse effects of glucocorticoid therapy for inner ear disorders. ORL. J. Otorhinolaryngol. Relat. Spec. 70(4), 271–4 (2008). doi:10.1159/000134381
G.S.G. Géléoc, J.R. Holt, Sound strategies for hearing restoration. Sci. 344(6184), 1241,062 (2014). doi:10.1126/science.1241062
A.F. Ghiz, A.N. Salt, J.E. DeMott, M.M. Henson, O.W. Henson, S.L. Gewalt, Quantitative anatomy of the round window and cochlear aqueduct in guinea pigs. Hear Res. 162(1–2), 105–12 (2001)
D. Greenwood, Comparing octaves, frequency ranges, and cochlear-map curvature across species. Hear Res. 94, 157–162 (1996)
D.S. Haynes, M. O’Malley , S. Cohen , K. Watford, R.F. Labadie, Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 117(1), 3–15 (2007). doi:10.1097/01.mlg.0000245058.11866.15
C. Himeno, M. Komeda, M. Izumikawa, K. Takemura, M. Yagi, Y. Weiping, T. Doi, H. Kuriyama, J. Miller, T. Yamashita, Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 167, 61–70 (2002)
M.C. Holley, Keynote review: The auditory system, hearing loss and potential targets for drug development. Drug Discov. Today. 10(19), 1269–82 (2005). doi:10.1016/S1359-6446(05)03595-6
J. Ito, T. Endo, T. Nakagawa, T. Kita, T.S. Kim, F. Iguchi, A new method for drug application to the inner ear. ORL. J. Otorhinolaryngol. Relat. Spec. 67(5), 272–5 (2005). doi:10.1159/000089407
K. Iwai, T. Nakagawa, T. Endo, Cochlear Protection by Local Insulin-Like Growth Factor-1 Application Using Biodegradable Hydrogel. Laryngoscope. 116(4), 529–533 (2006)
M. Izumikawa, R. Minoda, K. Kawamoto, Ka. Abrashkin, D.L. Swiderski, D.F. Dolan, D.E. Brough, Y. Raphael, Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 11(3), 271–6 (2005). doi:10.1038/nm1193
S.K. Juhn, Barrier Systems in the Inner Ear. Acta Otolaryngol. 105(s458), 79–83 (1988)
S.K. Juhn, L.P. Rybak, Labyrinthine Barriers and Cochlear Homeostasis. Acta Otolaryngol. 91(1–6), 529–534 (1981)
S.K. Juhn, B.A. Hunter, R.M. Odland, Blood-labyrinth barrier and fluid dynamics of the inner ear. Int. Tinnitus. J. 7(2), 72–83 (2001)
E.S. Kim, E. Gustenhoven, M.J. Mescher, E.E.L. Pararas, Ka. Smith, A.J. Spencer, V. Tandon, J.T. Borenstein, J. Fiering, A microfluidic reciprocating intracochlear drug delivery system with reservoir and active dose control. Lab Chip. 14(4), 710–21 (2014). doi:10.1039/c3lc51105g
G. Li, Frenz Da, S. Brahmblatt, J.G. Feghali, R.J. Ruben, D. Berggren, J. Arezzo, T.R. Van De Water, Round window membrane delivery of L-methionine provides protection from cisplatin ototoxicity without compromising chemotherapeutic efficacy. Neurotoxicology. 22(2), 163–76 (2001)
H. Li, G. Roblin, H. Liu, S. Heller, Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA. 100(23), 13,495–500 (2003). doi:10.1073/pnas.2334503100
H. Li, C.E. Corrales, A. Edge, S. Heller, Stem cells as therapy for hearing loss. Trends Mol. Med. 10(7), 309–15 (2004). doi:10.1016/j.molmed.2004.05.008
T. Littman, R.P. Bobbin, M. Fallon, J.L. Puel, The quinoxalinediones DNQX, CNQX and two relateed congeners suppress hair cell-to-auditory nerve transmission. Hear Res. 40, 45–54 (1989)
E.D. Lynch, J. Kil, Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov. Today. 10(19), 1291–1298 (2005)
Y. Maeda, A. Sheffield, R. Smith, Therapeutic regulation of gene expression in the inner ear using RNA interference. 13–36. 66 (2009). doi:10.1159/000218205.The
Aa. McCall, E.E.L. Swan, J.T. Borenstein, W.F. Sewell, S.G. Kujawa, M.J. McKenna, Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear. 31(2), 156–65 (2010). doi:10.1097/AUD.0b013e3181c351f2
M.J. Mescher, E.E.L. Swan, J. Fiering, M.E. Holmboe, W.F. Sewell, S.G. Kujawa, M.J. McKenna, J.T. Borenstein, Fabrication Methods and Performance of Low-Permeability Microfluidic Components for a Miniaturized Wearable Drug Delivery System. JMEMS. 18(3), 501–510 (2009)
K. Mizutari, M. Fujioka, M. Hosoya, N. Bramhall, H.J. Okano, H. Okano, A.S.B. Edge, Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 77(1), 58–69 (2013). doi:10.1016/j.neuron.2012.10.032
K. Murai, R. Tyler, L. Harker, J. Stouffer, Review of phramacologic treatment of tinnitus. Am. J. Otol. 13(5), 454–464 (1992)
F. Noushi, R. T. Richardson, J. Hardman, G. Clark, S. O’Leary, Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol. Neurotol. 26(3), 528–33 (2005)
K. Ohyama, A.N. Salt, R. Thalmann, Volume flow rate of perilymph in the guinea-pig cochlea. Hear Res. 35(2–3), 119–29 (1988)
E. Pararas, Z. Chen, J. Fiering, Kinetics of reciprocating drug delivery to the inner ear. J. Control. Release. 152(2), 270–277 (2011). doi:10.1016/j.jconrel.2011.02.021.Kinetics
T.N. Parks, The AMPA receptors of auditory neurons. Hear Res. 147(1-2), 77–91 (2000)
M. Praetorius, K. Baker, D.E. Brough, P. Plinkert, H. Staecker, Pharmacodynamics of adenovector distribution within the inner ear tissues of the mouse. Hear Res. 227(1–2), 53–8 (2007). doi:10.1016/j.heares.2006.07.002
R.T. Richardson, A. Wise, S. O’Leary, J. Hardman, D. Casley, G. Clark, Tracing neurotrophin-3 diffusion and uptake in the guinea pig cochlea. Hear Res. 198(1-2), 25–35 (2004). doi:10.1016/j.heares.2004.02.012
R.T. Richardson, F. Noushi, S. O’Leary, Inner ear therapy for neural preservation. Audiol. Neurootol. 11(6), 343–56 (2006). doi:10.1159/000095896
R.T. Richardson, A.K. Wise, B.C. Thompson, B.O. Flynn, P.J. Atkinson, N.J. Fretwell, J.B. Fallon, G.G. Wallace, R.K. Shepherd, G.M. Clark, S.J. O’Leary, Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 30(13), 2614–24 (2009). doi:10.1016/j.biomaterials.2009.01.015
L. Rybak, C. Whitworth, Ototoxicity: therapeutic opportunities. Drug Discov. Today. 10(19), 1313–1321 (2005)
A. Salt, Y. Ma, Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 154, 88–97 (2001)
A.N. Salt, S.K.R. Plontke, Local inner-ear drug delivery and pharmacokinetics. Drug Discov. Today. 10 (19), 1299–306 (2005). doi:10.1016/S1359-6446(05)03574-9
W. Sewell, in Neurotransmitters and synaptic transmission, ed. by P. Dallos, A.N. Popper, R.R. Fay. Cochlea Springer Handb. Audit. Res. Vol. 8, chap 9 (Springer, New York, New York, NY, 1996), pp. 503–533
W.F. Sewell, J.T. Borenstein, Z. Chen, J. Fiering, O. Handzel, M. Holmboe, E.S. Kim, S.G. Kujawa, M.J. McKenna, M.M. Mescher, B. Murphy, E.E.L. Swan, M. Peppi, S. Tao, Development of a microfluidics-based intracochlear drug delivery device. Audiol. Neurootol. 14(6), 411–22 (2009). doi:10.1159/000241898
Y. Shinomori, D. Spack, Volumetric and dimensional analysis of the guinea pig inner ear. Ann. Otol. Rhinol. Laryngol. 110(1), 91–98 (2001)
A. Shulman, B. Goldstein, Intratympanic drug therapy with steroids for tinnitus control: a preliminary report. Int. Tinnitus. J. 6(1), 10–20 (2000)
E.E.L. Swan, M.J. Mescher, W.F. Sewell, S.L. Tao, J.T. Borenstein, Inner ear drug delivery for auditory applications. Adv. Drug Deliv. Rev. 60(15), 1583–99 (2008). doi:10.1016/j.addr.2008.08.001
E.O. Thalen, H.P. Wit, J.M. Segenhout, F.W. Albers, Dynamics of inner ear pressure change caused by intracranial pressure manipulation in the guinea pig. Acta Otolaryngol. 121(4), 470–6 (2001)
M. Thorne, A.N. Salt, J.E. DeMott, M.M. Henson, O.W. Henson, S.L. Gewalt, Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 109(10), 1661–8 (1999). doi:10.1097/00005537-199910000-00021
M.M. Vio, R.H. Holme, Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov. Today. 10(00), 1263–1265 (2005)
W.R. Wilson, F.M. Byl, N. Laird, The Efficacy of Steroids in the Treatment of Idiopathic Sudden Hearing Loss: A Double-blind Clinical Study. Arch Otolaryngol - Head Neck Surg. 106(12), 772–776 (1980). doi:10.1001/archotol.1980.00790360050013
M. Yoshida, L.D. Lowry, Hydrostatic pressure measurement of endolymph and perilymph in the guinea pig cochlea. Am. J. Otolaryngol. 5(3), 159–65 (1984)
Acknowledgements
The project described was supported by Award Number R01DC006848 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute on Deafness and other Communication Disorders or the National Institutes of Health. We would also like to thank Transon Nguyen for his assistance with laser machining.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vishal Tandon and Woo Seok Kang contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Tandon, V., Kang, W., Spencer, A.J. et al. Microfabricated infuse-withdraw micropump component for an integrated inner-ear drug-delivery platform. Biomed Microdevices 17, 37 (2015). https://doi.org/10.1007/s10544-014-9923-8
Published:
DOI: https://doi.org/10.1007/s10544-014-9923-8