Abstract
Purpose
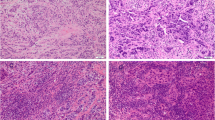
We studied the long-term outcomes of invasive micropapillary carcinoma (IMPCs) of the breast in relation to stromal tumor infiltrating lymphocytes (sTILs), prognostic biomarkers and clinicopathological features.
Methods
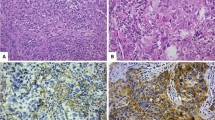
Stage I-III IMPCs treated with upfront surgery at our institution (January 2000 and December 2016) were included. Central pathology review was performed and sTILs (including zonal distribution and hot spot analysis) and tumor-associated plasma cells (TAPC) were evaluated. Expression of P53, BCL2, FOXP3, and WT1, which are variably linked to breast cancer prognosis, was measured by immunohistochemistry using tissue microarrays. Time-to-event endpoints were distant recurrence free interval (DRFI) and breast cancer-specific survival (BCSS).
Results
We included 111 patients of whom 59% were pure IMPCs. Standard clinicopathological features were comparable between pure and non-pure IMPCs. Overall, the mean sTILs level was 20% with higher proportion of sTILs present at the invasive front. There were no significant differences between pure- and non-pure IMPCs in sTILs levels, nor in the spatial distribution of the hot spot regions or in the distribution of TAPC. Higher sTILs correlated with worse DRFI (HR = 1.55; p = 0.0172) and BCSS (HR = 2.10; p < 0.001).
Conclusions
Clinicopathological features, geographical distribution of sTILs and TAPC are similar between pure and non-pure IMPCs. Despite a high proportion of grade 3 tumors and lymph node involvement, we observed a low rate of distant recurrences and breast cancer-related death in this cohort of stage I-III IMPCs treated with primary surgery. Caution in interpretation of the observed prognostic correlations is required given the very low number of events, warranting validation in other cohorts.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Marchiò C, Horlings HM, Vincent-Salomon (2019) Invasive micropapillary carcinoma. In: WHO classification of tumours of the breast, 5th edn. International agency for research on cancer, Lyon, France pp. 128–130.
Fisher ER, Palekar AS, Redmond C, Barton B, Fisher B (1980) Pathologic findings from the national surgical adjuvant breast project. Am J Clin Pathol 73:313–322
Marchiò C, Iravani M, Natrajan R, Lambros MB, Savage K, Tamber N et al (2008) Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J Pathol 2015:398–410
Chen L, Fan Y, Lang R, Guo X, Sun Y, Cui L et al (2008) Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol 16:155–163
Yang Y-L, Liu B-B, Zhang X, Fu L (2016) Invasive micropapillary carcinoma of the breast: an update. Arch Pathol Lab Med 140:799–805
Li G, Yang S, Yao J, Wang Z, Yao G, Liu M et al (2016) Invasive micropapillary carcinoma of the breast had poor clinical characteristics but showed no difference in prognosis compared with invasive ductal carcinoma. World J Surg Oncol 14:207
Chen AC, Paulino AC, Schwartz MR, Rodriguez AA, Bass BL, Chang JC et al (2014) Population-based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br J Cancer 111:619–622
Wu Y, Zhang N, Yang Q (2017) The prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in the breast: a meta-analysis. BMC Cancer 17:839
Hao S, Zhao Y-Y, Peng J-J, Ren F, Yang W-T, Yu K-D et al (2019) Invasive micropapillary carcinoma of the breast had no difference in prognosis compared with invasive ductal carcinoma: a propensity-matched analysis. Sci Rep 9:286
Liu Y, Huang X, Bi R, Yang W, Shao Z (2014) Similar prognoses for invasive micropapillary breast carcinoma and pure invasive ductal carcinoma: a retrospectively matched cohort study in China. PLoS ONE. https://doi.org/10.1371/journal.pone.0106564
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M et al (2019) Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 37:559–569
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs working group 2014. Ann Oncol 26:259–271
Guo X, Chen L, Lang R, Fan Y, Zhang X, Fu L (2006) Invasive micropapillary carcinoma of the breast: association of pathologic features with lymph node metastasis. Am J Clin Oncol 126:740–746
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL et al (2020) Estrogen and progesterone receptor testing in breast cancer: American society of clinical oncology/College of American pathologists guideline update. J Clin Oncol 38:1346–1366
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol 36:2105–2122
Brouckaert O, Laenen A, Vanderhaegen J, Wildiers H, Leunen K, Amant F et al (2012) Applying the 2011 St Gallen panel of prognostic markers on a large single hospital cohort of consecutively treated primary operable breast cancers. An Oncol 23:2578–2584
Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F et al (2018) Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the international immuno-oncology biomarker working group on breast cancer. Semin Cancer Biol 52:16–25
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy. J Clin Oncol 31:860–867
Bosisio FM, Wilmott JS, Volders N, Mercier M, Wouters J, Stas M et al (2016) Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod Pathol 29:347–358
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD et al (2017) QuPath: open source software for digital pathology image analysis. Sci Rep 7:16879
Berben L, Wildiers H, Marcelis L, Antoranz MA, Bosisio F, Hatse S et al (2020) Computerized scoring protocol for identification and quantification of different immune cell populations in breast tumor regions using QuPath software. Histopathology. https://doi.org/10.1111/his.14108
Altman DG, Bland JM (1983) Measurement in medicine: the analysis of method comparison studies. The Statistician 32:307–317
Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D et al (2015) Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the assessment of time-to-event endpoints in cancer trials). An Oncol 26:873–879
Fine JP, Gray RJA (1999) Proportional hazards model for the subdistribution of a competing risk. J Am Stat Soc 94:496–509
Paterakos M, Watkin WG, Edgerton SM, Moore DH, Thor AD (1999) Invasive micropapillary carcinoma of the breast: a prognostic study. Hum Pathol 30:1459–1463
Vingiani A, Maisonneuve P, Dell’orto P, Farante G, Rotmensz N, Lissidini G et al (2013) The clinical relevance of micropapillary carcinoma of the breast: a case-control study. Histopathology 63:217–224
Luna-Moré S, de los Santos F, Bretón JJ, Cañadas MA (1996) Estrogen and progesterone receptors, c-erbB-2, p53, and Bcl-2 in thirty-three invasive micropapillary breast carcinomas. Pathol Res Pract 192:27–32
Lewis GD, Xing Y, Haque W, Patel T, Schwartz M, Chen A et al (2019) Prognosis of lymphotropic invasive micropapillary breast carcinoma analyzed by using data from the national cancer database. Cancer Commun 39:60
Yu JI, Choi DH, Huh SJ, Cho EY, Kim K, Chie EK et al (2015) Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive ductal carcinoma of the breast: retrospective multicenter case-control study. Clin Breast cancer 15:353–361
Ye F, Yu P, Li N, Yang A, Xie X, Tang H et al (2020) Prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in breast: a meta-analysis of PSM studies. The Breast 51:11–20
Marchiò C, Iravani M, Natrajan R, Lambros MBK, Geyer FC, Savage K et al (2009) Mixed micropapillary-ductal carcinomas of the breast: a genomic and immunohistochemical analysis of morphologically distinct components. J Pathol 218:301–315
Pareja F, Selenica P, Brown DN, Sebastiao APM, da Silva EM, Da Cruz PA et al (2019) Micropapillary variant of mucinous carcinoma of the breast shows genetic alterations intermediate between those of mucinous carcinoma and micropapillary carcinoma. Histopathology 75:139–145
Jackson HW, Fischer JR, Zanotelli VRT, Ali HR, Mechera R, Soysal SD et al (2020) The single-cell pathology landscape of breast cancer. Nature 578:615–620
Nawaz S, Heindl A, Koelble K, Yan Y (2015) Beyond immune density: critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod Pathol 28:766–777
Heindl A, Sestak I, Naidoo K, Cuzick J, Dowsett M, Yuan Y (2018) Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER+ breast cancer. J Nat Cancer Inst 110:166–175
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. The Lancet Oncol 19:40–50
Guo X, Fan Y, Lang R, Gu F, Chen L, Cui L et al (2008) Tumor infiltrating lymphocytes differ in invasive micropapillary carcinoma and medullary carcinoma of breast. Mod Pathol 21:1101–1107
Dawson S-J, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J et al (2010) BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer 103:668–675
Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J et al (2006) Bcl-2 is a prognostic marker in breast cancer independently of the nottingham prognostic index. Clin Cancer Res 12:2468–2475
Seong M-K, Lee J-Y, Byeon J, Sohn Y-J, Seol H, Lee J-K et al (2015) Bcl-2 is a highly significant prognostic marker of hormone-receptor-positive, human epidermal growth factor receptor-2-negative breast cancer. Breast Cancer Res Treat 150:141–148
Bhargava V, Kell DL (1994) Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. Am J Pathol 145:535–540
Leek RD, Kaklamanis L, Pezzella F, Gatter KC, Harris AL (1994) bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer 69:135–139
Doglioni C, Dei Tos AP, Laurino L, Chiarelli C, Barbareschi M, Viale G (1994) The prevalence of BCL-2 immunoreactivity in breast carcinomas and its clinicopathological correlates, with particular reference to oestrogen receptor status. Virchows Arch 424:47–51
Boyle DP, McArt DG, Irwin G, Wilhelm-Benartzi CS, Lioe TF, Sebastian E et al (2014) The prognostic significance of the aberrant extremes of p53 immunophenotypes in breast cancer. Histopathology 65:340–352
Dumay A, Feugeas J-P, Wittmer E, Lehmann-Che J, Bertheau P, Espié M et al (2013) Distinct tumor protein p53 mutants in breast cancer subgroups. Int J Cancer 132:1227–1231
Douglass S, Ali S, Meeson AP, Browell D, Kirby JA (2012) The role of FOXP3 in the development and metastatic spread of breast cancer. Cancer Metastasis Rev 31:843–854
Lee AHS, Paish EC, Marchio C, Sapino A, Schmitt FC, Ellis IO et al (2007) The expression of Wilms’ tumour-1 and Ca125 in invasive micropapillary carcinoma of the breast. Histopathology 51:824–828
Domfeh AB, Carley AL, Striebel JM, Karabakhtsian RG, Florea AV, McManus K et al (2008) WT1 immunoreactivity in breast carcinoma: selective expression in pure and mixed mucinous subtypes. Mod Pathol 21:1217–1223
Singh K, Hanley LC, Sung CJ, Quduus MR (2019) Comparison of PAX8 expression in breast carcinoma using MRQ50 and BC12 monoclonal antibodies. Appl Immunohistol. https://doi.org/10.1097/PAI.0000000000000796
Silberstein GB, Van Horn K, Strickland P, Roberts CT, Daniel CW (1997) Altered expression of the WT1 wilms tumor suppressor gene in human breast cancer. Proc Natl Acad Sci USA 94:8132–8137
Zapata-Benavides P, Tuna M, Lopez-Berestein G, Tari AM (2002) Downregulation of Wilms’ tumor 1 protein inhibits breast cancer proliferation. Biochm Biophys Res Commun 295:784–790
Buisseret L, Desmedt C, Garaud S, Fornili M, Wang X, Van den Eyden G et al (2017) Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER+ breast cancer. Mod Pathol 30:1204–1212
Eom YH, Kim HS, Lee A, Song BJ, Chae BJ (2016) BCL2 as a subtype-specific prognostic marker for breast cancer. J Breast Cancer 19:252–260
Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T et al (2013) FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol 1:625–632
Author information
Authors and Affiliations
Contributions
Conception and design: GF, KP; Development of methodology: FD, KP, GF; Acquisition of data: FD, KP; Analysis and interpretation of data: FD, KP, GF, AL; Writing, review and/or revision of the manuscript: all authors; Administrative, technical, or material support: GF; Study supervision: GF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Approval was obtained from the ethics committee of KU Leuven ethics committee (ref. n° MP002896).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deman, F., Punie, K., Laenen, A. et al. Assessment of stromal tumor infiltrating lymphocytes and immunohistochemical features in invasive micropapillary breast carcinoma with long-term outcomes. Breast Cancer Res Treat 184, 985–998 (2020). https://doi.org/10.1007/s10549-020-05913-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05913-x