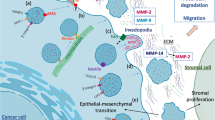
Abstract
Rapidly increasing scientific reports of exosomes and their biological effects have improved our understanding of their cellular sources and their cell-to-cell communication. These nano-sized vesicles act as potent carriers of regulatory bio-macromolecules and can induce regulatory functions by delivering them from its source to recipient cells. The details of their communication network are less understood. Recent studies have shown that apart from delivering its cargo to the cells, it can directly act on extracellular matrix (ECM) proteins and growth factors and can induce various remodeling events. More importantly, exosomes carry many surface-bound proteases, which can cleave different ECM proteins and carbohydrates and can shed cell surface receptors. These local extracellular events can modulate signaling cascades, which consequently influences the whole tissue and organ. This review aims to highlight the critical roles of exosomal proteases and their mechanistic insights within the cellular and extracellular environment.

Similar content being viewed by others
References
Théry, C., Zitvogel, L., & Amigorena, S. (2002). Exosomes: Composition, biogenesis and function. Nature Reviews. Immunology, 2(8), 569–579.
Nawaz, M., et al. (2018). Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells, 7(10), 167.
Li, S. P., Lin, Z. X., Jiang, X. Y., & Yu, X. Y. (2018). Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacologica Sinica, 39(4), 542–551.
Elsherbini, A., & Bieberich, E. (2018). Ceramide and Exosomes: A Novel Target in Cancer Biology and Therapy (Vol. 140). Elsevier Ltd.
Williams, C., et al. (2018). Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives. Journal of Extracellular Vesicles, 7(1).
Williams, C., et al. (2019). Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Scientific Reports, 9(1), 11920.
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., & Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659.
Q. Liu et al., “Donor dendritic cell–derived exosomes promote allograft-targeting immune response,” vol. 126, no. 8, pp. 2805–2820, 2016.
Fabbri, M., Paone, A., Calore, F., Galli, R., Croce, C. M., & Mediators, C. C. (2013). A new role for microRNAs, as ligands of Toll-like receptors Muller. RNA Biology, 10(2, no. February), 169–174.
Thakur, B. K., Zhang, H., Becker, A., Matei, I., Huang, Y., Costa-Silva, B., Zheng, Y., Hoshino, A., Brazier, H., Xiang, J., Williams, C., Rodriguez-Barrueco, R., Silva, J. M., Zhang, W., Hearn, S., Elemento, O., Paknejad, N., Manova-Todorova, K., Welte, K., Bromberg, J., Peinado, H., & Lyden, D. (2014). Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Research, 24(6), 766–769.
Kalluri, R., & Lebleu, V. S. (2016). Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harbor Symposia on Quantitative Biology, 81(1), 275–280.
Dourado, M. R., et al. (2019). Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. Journal of Extracellular Vesicles, 8(1).
Hamam, D., Abdouh, M., Gao, Z. H., Arena, V., Arena, M., & Arena, G. O. (2016). Transfer of malignant trait to BRCA1 deficient human fibroblasts following exposure to serum of cancer patients. Journal of Experimental & Clinical Cancer Research, 35(1), 1–12.
Minciacchi, V. R., et al. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Seminars in Cell & Developmental Biology, 40, 41–51.
McKelvey, K. J., Powell, K. L., Ashton, A. W., Morris, J. M., & McCracken, S. A. (2015). Exosomes: Mechanisms of uptake. Journal of Circulating Biomarkers, 4, 1–9.
Gonda, A., Kabagwira, J., Senthil, G. N., & Wall, N. R. (2019). Internalization of exosomes through receptor-mediated endocytosis. Molecular Cancer Research, 17(2), 337–347.
Maas, S. L. N., Breakefield, X. O., & Weaver, A. M. (2017). Extracellular vesicles: Unique intercellular delivery vehicles. Trends in Cell Biology, 27(3), 172–188.
Duijvesz, D., et al. (2013). Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer. PLoS One, 8(12), 1–10.
Huang, T., & Deng, C. X. (2019). Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. International Journal of Biological Sciences, 15(1), 1–11.
Shimoda, M., & Khokha, R. (2017). Metalloproteinases in extracellular vesicles. Biochimica et Biophysica Acta, Molecular Cell Research, 1864(11), 1989–2000.
Shimoda, M., & Khokha, R. (2013). Proteolytic factors in exosomes. Proteomics, 13(10–11), 1624–1636.
Das, A., Monteiro, M., Barai, A., Kumar, S., & Sen, S. (2017). MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Scientific Reports, 7(1), 1–13.
Das, S. S. A., Kapoor, A., Mehta, G. D., & Ghosh, S. K. (2013). Extracellular Matrix Density Regulates Extracellular Proteolysis via Modulation of Cellular Contractility. Journal of Carcinogenesis and Mutagenesis, S13.
Kapoor, A., Barai, A., Thakur, B., Das, A., Patwardhan, S. R., Monteiro, M., Gaikwad, S., Bukhari, A. B., Mogha, P., Majumder, A., de, A., Ray, P., & Sen, S. (2018). Soft drug-resistant ovarian cancer cells migrate via two distinct mechanisms utilizing myosin II-based contractility. Biochimica et Biophysica Acta, Molecular Cell Research, 1865(2), 392–405.
A. Haage and I. C. Schneider, “Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells,” pp. 1–11, 2014.
Gong, Y., Chippada-Venkata, U. D., & Oh, W. K. (2014). Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel), 6(3), 1298–1327.
Jiao, Y., et al. (2012). Matrix metalloproteinase-2 promotes αvβ3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PLoS One, 7(7), e41591.
Ginestra, A., Monea, S., Seghezzi, G., Dolo, V., Nagase, H., Mignatti, P., & Vittorelli, M. L. (1997). Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. The Journal of Biological Chemistry, 272(27), 17216–17222.
Sung, B. H., & Weaver, A. M. (2017). Exosome secretion promotes chemotaxis of cancer cells. Cell Adhesion & Migration, 11(2), 187–195.
Dolo, V., et al. (1998). Selective localization of matrix metalloproteinase 9, β 1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Research, 58(19), 4468–4474.
van der Vorst, E. P. C., de Jong, R. J., & Donners, M. M. P. C. (2018). Message in a Microbottle: Modulation of Vascular Inflammation and Atherosclerosis by Extracellular Vesicles. Frontiers in Cardiovascular Medicine, 5(January), 1–8.
Li, H., Qiu, Z., Li, F., & Wang, C. (2017). The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncology Letters, 14(5), 5865–5870.
Minciacchi, V. R., Freeman, M. R., & Di Vizio, D. (2015). Extracellular vesicles in Cancer: Exosomes, microvesicles and the emerging role of large Oncosomes. Seminars in Cell & Developmental Biology, 40, 41–51.
Runz, S., Keller, S., Rupp, C., Stoeck, A., Issa, Y., Koensgen, D., Mustea, A., Sehouli, J., Kristiansen, G., & Altevogt, P. (2007). Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecologic Oncology, 107(3), 563–571.
Han, K. Y., Dugas-Ford, J., Seiki, M., Chang, J. H., & Azar, D. T. (2015). Evidence for the involvement of MMP14 in MMP2 processing and recruitment in exosomes of corneal fibroblasts. Investigative Ophthalmology and Visual Science, 56(9), 5323–5329.
Hakulinen, J., Sankkila, L., Sugiyama, N., Lehti, K., & Keski-Oja, J. (2008). Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. Journal of Cellular Biochemistry, 105(5), 1211–1218.
Meng, W., Hao, Y., He, C., Li, L., & Zhu, G. (2019). Exosome-orchestrated hypoxic tumor microenvironment. Molecular Cancer, 18(1), 1–14.
Sung, B. H., Ketova, T., Hoshino, D., Zijlstra, A., & Weaver, A. M. (2015). Directional cell movement through tissues is controlled by exosome secretion. Nature Communications, 6(May), 1–14.
Shan, Y., et al. (2018). Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases. Cell Death & Disease, 9(3).
Groth, E., Pruessmeyer, J., Babendreyer, A., Schumacher, J., Pasqualon, T., Dreymueller, D., Higashiyama, S., Lorenzen, I., Grötzinger, J., Cataldo, D., & Ludwig, A. (2016). Stimulated release and functional activity of surface expressed metalloproteinase ADAM17 in exosomes. Biochimica et Biophysica Acta, Molecular Cell Research, 1863(11), 2795–2808.
Stoeck, A., Keller, S., Riedle, S., Sanderson, M. P., Runz, S., le Naour, F., Gutwein, P., Ludwig, A., Rubinstein, E., & Altevogt, P. (2006). A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. The Biochemical Journal, 393(3), 609–618.
Wanger, T. M., Dewitt, S., Collins, A., Maitland, N. J., Poghosyan, Z., & Knäuper, V. (2015). Differential regulation of TROP2 release by PKC isoforms through vesicles and ADAM17. Cellular Signalling, 27(7), 1325–1335.
Zaman, S., Jadid, H., Denson, A. C., & Gray, J. E. (2019). Targeting trop-2 in solid tumors: Future prospects. OncoTargets and Therapy, 12, 1781–1790.
Tugutova, E. A., Tamkovich, S. N., Patysheva, M. R., Afanas’ev, S. G., Tsydenova, A. A., Grigor’eva, A. E., Kolegova, E. S., Kondakova, I. V., & Yunusova, N. V. (2019). Relation between tetraspanin- associated and tetraspanin- non- associated exosomal proteases and metabolic syndrome in colorectal cancer patients. Asian Pacific Journal of Cancer Prevention, 20(3), 809–815.
Shimoda, M., Principe, S., Jackson, H. W., Luga, V., Fang, H., Molyneux, S. D., Shao, Y. W., Aiken, A., Waterhouse, P. D., Karamboulas, C., Hess, F. M., Ohtsuka, T., Okada, Y., Ailles, L., Ludwig, A., Wrana, J. L., Kislinger, T., & Khokha, R. (2014). Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nature Cell Biology, 16(9), 889–901.
Hansen, H. P., et al. (2016). CD30 on extracellular vesicles from malignant Hodgkin cells supports damaging of CD30 ligand-expressing bystander cells with Brentuximab-Vedotin, in vitro. Oncotarget, 7(21), 30523–30535.
Tauro, B. J., Mathias, R. A., Greening, D. W., Gopal, S. K., Ji, H., Kapp, E. A., Coleman, B. M., Hill, A. F., Kusebauch, U., Hallows, J. L., Shteynberg, D., Moritz, R. L., Zhu, H. J., & Simpson, R. J. (2013). Oncogenic H-Ras reprograms madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Molecular & Cellular Proteomics, 12(8), 2148–2159.
Yoneyama, T., Gorry, M., Sobo-Vujanovic, A., Lin, Y., Vujanovic, L., Gaither-Davis, A., Moss, M. L., Miller, M. A., Griffith, L. G., Lauffenburger, D. A., Stabile, L. P., Herman, J., & Vujanovic, N. L. (2018). ADAM10 sheddase activity is a potential lung-cancer biomarker. Journal of Cancer, 9(14), 2559–2570.
Yang, M., Li, Y., Chilukuri, K., Brady, O. A., Boulos, M. I., Kappes, J. C., & Galileo, D. S. (2011). L1 stimulation of human glioma cell motility correlates with FAK activation. Journal of Neuro-Oncology, 105(1), 27–44.
Tamkovich, S. N., Yunusova, N. V., Tugutova, E., Somov, A. K., Proskura, K. V., Kolomiets, L. A., Stakheyeva, M. N., Grigor’eva, A. E., Laktionov, P. P., & Kondakova, I. V. (2019). Protease cargo in circulating exosomes of breast cancer and ovarian cancer patients. Asian Pacific Journal of Cancer Prevention, 20(1), 255–262.
Lee, H. D., Koo, B. H., Kim, Y. H., Jeon, O. H., & Kim, D. S. (2012). Exosome release of ADAM15 and the functional implications of human macrophage-derived ADAM15 exosomes. The FASEB Journal, 26(7), 3084–3095.
Webber, J., Stone, T. C., Katilius, E., Smith, B. C., Gordon, B., Mason, M. D., Tabi, Z., Brewis, I. A., & Clayton, A. (2014). Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (somascantm) platform. Molecular & Cellular Proteomics, 13(4), 1050–1064.
Kato, T., et al. (2014). Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Research & Therapy, 16(4), 1–11.
Rana, S., Malinowska, K., & Zöller, M. (2013). Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia (United States), 15(3), 281–295.
McAtee, C. O., et al. (2019). Prostate tumor cell exosomes containing hyaluronidase Hyal1 stimulate prostate stromal cell motility by engagement of FAK-mediated integrin signaling. Matrix Biology, 78–79, 165–179.
Hong, Y., et al. (2018). Exosome as a vehicle for delivery of membrane protein therapeutics, PH20, for enhanced tumor penetration and antitumor efficacy. Advanced Functional Materials, 28(5), 1–9.
Genschmer, K. R., et al. (2019). Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell, 176(1–2), 113–126.e15.
Bulloj, A., Leal, M. C., Xu, H., Castaño, E. M., & Morelli, L. (2010). Insulin-degrading enzyme sorting in exosomes: A secretory pathway for a key brain amyloid-β degrading protease. Journal of Alzheimer's Disease, 19(1), 79–95.
Sanderson, R. D., Bandari, S. K., & Vlodavsky, I. (2019). Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling. Matrix Biology, 75–76, 160–169.
Thompson, C. A., Purushothaman, A., Ramani, V. C., Vlodavsky, I., & Sanderson, R. D. (2013). Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. The Journal of Biological Chemistry, 288(14), 10093–10099.
Murayama, T., Kataoka, H., Koita, H., Nabeshima, K., & Koono, M. (1991). Glycocalyceal bodies in a human rectal carcinoma cell line and their interstitial collagenolytic activities. Virchows Archiv. B, Cell Pathology Including Molecular Pathology, 60(1), 263–270.
Harris, D. A., Patel, S. H., Gucek, M., Hendrix, A., Westbroek, W., & Taraska, J. W. (2015). Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One, 10(3), 1–18.
Cocucci, E., Racchetti, G., & Meldolesi, J. (2009). Shedding microvesicles: Artefacts no more. Trends in Cell Biology, 19(2), 43–51.
Whiteside, T. L. (2016). Tumor-Derived Exosomes and Their Role in Cancer Progression (Vol. 74, 1st ed.). Elsevier Inc..
Edwards, D. R., Handsley, M. M., & Pennington, C. J. (2009). The ADAM metalloproteinases. Molecular Aspects of Medicine, 29(5), 258–289.
Wetzel, S., Seipold, L., & Saftig, P. (2017). The metalloproteinase ADAM10: A useful therapeutic target? Biochimica et Biophysica Acta, Molecular Cell Research, 1864(11), 2071–2081.
Levin, M., Udi, Y., Solomonov, I., & Sagi, I. (2017). Next generation matrix metalloproteinase inhibitors — Novel strategies bring new prospects. Biochimica et Biophysica Acta, Molecular Cell Research, 1864(11), 1927–1939.
Thathiah, A., Blobel, C. P., & Carson, D. D. (2003). Tumor necrosis factor-α converting enzyme/ADAM 17 mediates MUC1 shedding. The Journal of Biological Chemistry, 278(5), 3386–3394.
Gooz, M. (2010). ADAM-17: The enzyme that does it all. Critical Reviews in Biochemistry and Molecular Biology, 45(2), 146–169.
Mishra, H. K., Ma, J., & Walcheck, B. (2017). Ectodomain shedding by ADAM17: Its ole in neutrophil recruitment and the Impairment of this process during sepsis. Frontiers in Cellular and Infection Microbiology, 7(APR), 1–10.
Lambrecht, B. N., Vanderkerken, M., & Hammad, H. (2018). The emerging role of ADAM metalloproteinases in immunity. Nature Reviews. Immunology, 18(12), 745–758.
Chalaris, A., Adam, N., Sina, C., Rosenstiel, P., Lehmann-Koch, J., Schirmacher, P., Hartmann, D., Cichy, J., Gavrilova, O., Schreiber, S., Jostock, T., Matthews, V., Häsler, R., Becker, C., Neurath, M. F., Reiß, K., Saftig, P., Scheller, J., & Rose-John, S. (2010). Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. The Journal of Experimental Medicine, 207(8), 1617–1624.
Hartmann, D. (2002). The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Human Molecular Genetics, 11(21), 2615–2624.
Purow, B. (2012). Notch signaling in embryology and Cancer. Advances in Experimental Medicine and Biology, 727, 174–315.
Mullooly, M., McGowan, P. M., Kennedy, S. A., Madden, S. F., Crown, J., O' Donovan, N., & Duffy, M. J. (2015). ADAM10: A new player in breast cancer progression? British Journal of Cancer, 113(6), 945–951.
Li, B. X., et al. (2011). Effects of RNA interference-mediated gene silencing of JMJD2A on human breast cancer cell line MDA-MB-231 in vitro. Journal of Experimental & Clinical Cancer Research, 30(1), 1–9.
Feldinger, K., Generali, D., Kramer-Marek, G., Gijsen, M., Ng, T. B., Wong, J. H., Strina, C., Cappelletti, M., Andreis, D., Li, J. L., Bridges, E., Turley, H., Leek, R., Roxanis, I., Capala, J., Murphy, G., Harris, A. L., & Kong, A. (2014). ADAM10 mediates trastuzumab resistance and is correlated with survival in HER2 positive breast cancer. Oncotarget, 5(16), 6633–6646.
Wozniak, J., & Ludwig, A. (2018). Novel role of APP cleavage by ADAM10 for breast cancer metastasis. EBioMedicine, 38, 5–6.
Duffy, M. J., et al. (2011). The ADAMs family of proteases: New biomarkers and therapeutic targets for cancer? Clinical Proteomics, 8(1), 1–13.
“The ADAMTS metalloproteinases,” Biochem. J., vol. 27, pp. 15–27, 2011.
Cal, S., & López-Otín, C. (2015). ADAMTS proteases and cancer. Matrix Biology, 44–46, 77–85.
Kelwick, R., Desanlis, I., Wheeler, G. N., & Edwards, D. R. (2015). The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biology, 16(1).
Mead, T. J., du, Y., Nelson, C. M., Gueye, N. A., Drazba, J., Dancevic, C. M., Vankemmelbeke, M., Buttle, D. J., & Apte, S. S. (2018). ADAMTS9-regulated Pericellular matrix dynamics governs focal adhesion-dependent smooth muscle differentiation. Cell Reports, 23(2), 485–498.
El-Safory, N. S., Fazary, A. E., & Lee, C. K. (2010). Hyaluronidases, a group of glycosidases: Current and future perspectives. Carbohydrate Polymers, 81(2), 165–181.
McAtee, C. O., Barycki, J. J., & Simpson, M. A. (2014). Emerging roles for hyaluronidase in cancer metastasis and therapy. Advances in Cancer Research, 123(402), 1–34.
Josefsson, A., Adamo, H., Hammarsten, P., Granfors, T., Stattin, P., Egevad, L., Laurent, A. E., Wikström, P., & Bergh, A. (2011). Prostate cancer increases hyaluronan in surrounding nonmalignant stroma, and this response is associated with tumor growth and an unfavorable outcome. The American Journal of Pathology, 179(4), 1961–1968.
Tan, J. X., et al. (2011). Upregulation of HYAL1 expression in breast cancer promoted tumor cell proliferation, migration, invasion and angiogenesis. PLoS One, 6(7).
Kikuchi, S., Yoshioka, Y., Prieto-Vila, M., & Ochiya, T. (2019). Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. International Journal of Molecular Sciences, 20(10), 1–17.
Shao, C., et al. (2018). Role of hypoxia-induced exosomes in tumor biology. Molecular Cancer, 17(1), 1–8.
Lee, J. K., et al. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One, (8, 12).
Gopal, S. K., Greening, D. W., Hanssen, E. G., Zhu, H. J., Simpson, R. J., & Mathias, R. A. (2016). Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget, 7(15), 19709–19722.
Poggio, M., et al. (2019). Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell, 177(2), 414–427.e13.
Zeng, Z., et al. (2018). Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature Communications, 9(1).
Tang, M. K. S., et al. (2018). Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nature Communications, 9(1), 1–15.
Mao, Y., Keller, E. T., Garfield, D. H., Shen, K., & Wang, J. (2013). Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Reviews, 32(1–2), 303–315.
Ludwig, N., & Whiteside, T. L. (2018). Potential roles of tumor-derived exosomes in angiogenesis. Expert Opinion on Therapeutic Targets, 22(5), 409–417.
Paggetti, J., Haderk, F., Seiffert, M., Janji, B., Distler, U., Ammerlaan, W., Kim, Y. J., Adam, J., Lichter, P., Solary, E., Berchem, G., & Moussay, E. (2015). Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood, 126(9), 1106–1117.
Corrado, C., Saieva, L., Raimondo, S., Santoro, A., De Leo, G., & Alessandro, R. (2016). Chronic myelogenous leukaemia exosomes modulate bone marrow microenvironment through activation of epidermal growth factor receptor. Journal of Cellular and Molecular Medicine, 20(10), 1829–1839.
Javidi-Sharifi, N., et al. (2019). Fgf2-fgfr1 signaling regulates release of leukemia-protective exosomes from bone marrow stromal cells. Elife, 8, 1–23.
Purushothaman, A., Bandari, S. K., Liu, J., Mobley, J. A., Brown, E. A., & Sanderson, R. D. (2016). Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. The Journal of Biological Chemistry, 291(4), 1652–1663.
Yang, L., & Zhang, Y. (2017). Tumor-associated macrophages: from basic research to clinical application. Journal of Hematology & Oncology, 10(1), 58.
Zhang, W., Zhang, J., Cheng, L., Ni, H., You, B., Shan, Y., Bao, L., Wu, D., Zhang, T., Yue, H., & Chen, J. (2018). A disintegrin and metalloprotease 10-containing exosomes derived from nasal polyps promote angiogenesis and vascular permeability. Molecular Medicine Reports, 17(4), 5921–5927.
Chen, W., Xiao, M., Zhang, J., & Chen, W. (2018). M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research, 37(1), 1–15.
Plebanek, M. P., et al. (2017). Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nature Communications, 8(1).
Paszek, M. J., Zahir, N., Johnson, K. R., Lakins, J. N., Rozenberg, G. I., Gefen, A., Reinhart-King, C. A., Margulies, S. S., Dembo, M., Boettiger, D., Hammer, D. A., & Weaver, V. M. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell, 8(3), 241–254.
Lu, P., Weaver, V. M., & Werb, Z. (Feb. 2012). The extracellular matrix: A dynamic niche in cancer progression. The Journal of Cell Biology, 196(4), 395–406.
Kumar, S., Das, A., & Sen, S. (2018). Multicompartment cell-based modeling of confined migration: Regulation by cell intrinsic and extrinsic factors. Molecular Biology of the Cell, 29(13), 1599–1610.
Das, A., Barai, A., Monteiro, M., Kumar, S., & Sen, S. (2019). Nuclear softening is essential for protease-independent migration. Matrix Biology, 82, 4–19.
Petrova, V., Annicchiarico-Petruzzelli, M., Melino, G., & Amelio, I. (2018). The hypoxic tumour microenvironment. Oncogenesis, 7(1).
de Jong, O. G., van Balkom, B. W. M., Gremmels, H., & Verhaar, M. C. (2016). Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. Journal of Cellular and Molecular Medicine, 20(2), 342–350.
Li, R., et al. (2019). Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Molecular Cancer, 18(1), 1–19.
Hoshino, D., Kirkbride, K. C., Costello, K., Clark, E. S., Sinha, S., Grega-Larson, N., Tyska, M. J., & Weaver, A. M. (2013). Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Reports, 5(5), 1159–1168.
Fu, M., Gu, J., Jiang, P., Qian, H., Xu, W., & Zhang, X. (2019). Exosomes in gastric cancer: Roles, mechanisms, and applications. Molecular Cancer, 18(1), 1–12.
Zhang, W., Gu, J., Chen, J., Zhang, P., Ji, R., Qian, H., Xu, W., & Zhang, X. (2017). Interaction with neutrophils promotes gastric cancer cell migration and invasion by inducing epithelial-mesenchymal transition. Oncology Reports, 38(5), 2959–2966.
Chen, L., et al. (2018). Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA. Nature Communications, 9(1).
Fedele, C., Singh, A., Zerlanko, B. J., Iozzo, R. V., & Languino, L. R. (2015). The alphavbeta6 integrin is transferred Intercellularly via exosomes. The Journal of Biological Chemistry, 290(8), 4545–4551.
Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V., & Wargo, J. A. (2019). The microbiome, cancer, and cancer therapy. Nature Medicine, 25(3), 377–388.
Urbaniak, C., Gloor, G. B., Brackstone, M., Scott, L., Tangney, M., & Reida, G. (2016). The microbiota of breast tissue and its association with breast cancer. Applied and Environmental Microbiology, 82(16), 5039–5048.
Wei, M. Y., et al. (2019). The microbiota and microbiome in pancreatic cancer: More influential than expected. Molecular Cancer, 18(1), 1–15.
L. T. Geller et al., “Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine Leore T. Geller,1* Michal Barzily-Rokni,2* Tal Danino,3† Oliver H. Jonas,4,5 Noam Shental,6 Deborah Nejman,1 Nancy Gavert,1 Yaara Zwang,1 Zachary ,” vol. 1160, no. September, pp. 1156–1160, 2017.
Pushalkar, S., Hundeyin, M., Daley, D., Zambirinis, C. P., Kurz, E., Mishra, A., Mohan, N., Aykut, B., Usyk, M., Torres, L. E., Werba, G., Zhang, K., Guo, Y., Li, Q., Akkad, N., Lall, S., Wadowski, B., Gutierrez, J., Kochen Rossi, J. A., Herzog, J. W., Diskin, B., Torres-Hernandez, A., Leinwand, J., Wang, W., Taunk, P. S., Savadkar, S., Janal, M., Saxena, A., Li, X., Cohen, D., Sartor, R. B., Saxena, D., & Miller, G. (2018). The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discovery, 8(4), 403–416.
Schwechheimer, C., & Kuehn, M. J. (2015). Outer-membrane vesicles from gram-negative bacteria: Biogenesis and functions. Nature Reviews. Microbiology, 13(10), 605–619.
Yu, Y. J., Wang, X. H., & Fan, G. C. (2018). Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacologica Sinica, 39(4), 514–533.
Sieber, K. B., Bromley, R. E., & Dunning Hotopp, J. C. (2017). Lateral gene transfer between prokaryotes and eukaryotes. Experimental Cell Research, 358(2), 421–426.
Chang, A. H., & Parsonnet, J. (2010). Role of bacteria in oncogenesis. Clinical Microbiology Reviews, 23(4), 837–857.
Robinson, K. M., Crabtree, J., Mattick, J. S. A., Anderson, K. E., & Hotopp, J. C. D. (2017). Distinguishing potential bacteria-tumor associations from contamination in a secondary data analysis of public cancer genome sequence data. Microbiome, 5(1), 1–17.
Surve, M. V., et al. (2016). Membrane vesicles of group B Streptococcus disrupt Feto-maternal barrier leading to preterm birth. PLoS Pathogens, 12(9), 1–23.
Barteneva, N. S., Baiken, Y., Fasler-Kan, E., Alibek, K., Wang, S., Maltsev, N., Ponomarev, E. D., Sautbayeva, Z., Kauanova, S., Moore, A., Beglinger, C., & Vorobjev, I. A. (2017). Extracellular vesicles in gastrointestinal cancer in conjunction with microbiota: On the border of kingdoms. Biochimica et Biophysica Acta, Reviews on Cancer, 1868(2), 372–393.
Housman, G., Byler, S., Heerboth, S., Lapinska, K., Longacre, M., Snyder, N., & Sarkar, S. (2014). Drug resistance in cancer: An overview. Cancers (Basel), 6(3), 1769–1792.
Zhang, J., Gu, Y., & Chen, B. (2019). Mechanisms of drug resistance in acute myeloid leukemia. OncoTargets and Therapy, 12, 1937–1945.
Bandari, S. K., Purushothaman, A., Ramani, V. C., Brinkley, G. J., Chandrashekar, D. S., Varambally, S., Mobley, J. A., Zhang, Y., Brown, E. E., Vlodavsky, I., & Sanderson, R. D. (2018). Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biology, 65(2018), 104–118.
Vlodavsky, I., Gross-Cohen, M., Weissmann, M., Ilan, N., & Sanderson, R. D. (2018). Opposing functions of Heparanase-1 and Heparanase-2 in Cancer progression. Trends in Biochemical Sciences, 43(1), 18–31.
Kamerkar, S., LeBleu, V. S., Sugimoto, H., Yang, S., Ruivo, C. F., Melo, S. A., Lee, J. J., & Kalluri, R. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature, 546(7659), 498–503.
Dimou, A., Syrigos, K. N., & Saif, M. W. (2012). Overcoming the stromal barrier: Technologies to optimize drug delivery in pancreatic cancer. Therapeutic Advances in Medical Oncology, 4(5), 271–279.
Hoshino, A., Costa-Silva, B., Shen, T. L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., Molina, H., Kohsaka, S., di Giannatale, A., Ceder, S., Singh, S., Williams, C., Soplop, N., Uryu, K., Pharmer, L., King, T., Bojmar, L., Davies, A. E., Ararso, Y., Zhang, T., Zhang, H., Hernandez, J., Weiss, J. M., Dumont-Cole, V. D., Kramer, K., Wexler, L. H., Narendran, A., Schwartz, G. K., Healey, J. H., Sandstrom, P., Jørgen Labori, K., Kure, E. H., Grandgenett, P. M., Hollingsworth, M. A., de Sousa, M., Kaur, S., Jain, M., Mallya, K., Batra, S. K., Jarnagin, W. R., Brady, M. S., Fodstad, O., Muller, V., Pantel, K., Minn, A. J., Bissell, M. J., Garcia, B. A., Kang, Y., Rajasekhar, V. K., Ghajar, C. M., Matei, I., Peinado, H., Bromberg, J., & Lyden, D. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527(7578), 329–335.
Sagar, G., et al. (2017). Pathogenesis of pancreatic Cancer exosome-induced lipolysis in adipose tissue. Gut, 65(7), 1165–1174.
Zhou, M., Chen, J., Zhou, L., Chen, W., Ding, G., & Cao, L. (2014). Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cellular Immunology, 292(1–2), 65–69.
Li, Z., Jiang, P., Li, J., Peng, M., Zhao, X., Zhang, X., Chen, K., Zhang, Y., Liu, H., Gan, L., Bi, H., Zhen, P., Zhu, J., & Li, X. (2018). Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene, 37(28), 3822–3838.
Wang, X., Luo, G., Zhang, K., Cao, J., Huang, C., Jiang, T., Liu, B., Su, L., & Qiu, Z. (2018). Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kg to promote pancreatic cancer metastasis. Cancer Research, 78(16), 4586–4598.
Chiba, M., Kubota, S., Sato, K., & Monzen, S. (2018). Exosomes released from pancreatic cancer cells enhance angiogenic activities via dynamin-dependent endocytosis in endothelial cells in vitro. Scientific Reports, 8(1), 1–9.
Melo, S. A., Luecke, L. B., Kahlert, C., Fernandez, A. F., Gammon, S. T., Kaye, J., LeBleu, V. S., Mittendorf, E. A., Weitz, J., Rahbari, N., Reissfelder, C., Pilarsky, C., Fraga, M. F., Piwnica-Worms, D., & Kalluri, R. (2015). Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature, 523(7559), 177–182.
Lau, C., et al. (2013). Role of pancreatic cancer-derived exosomes in salivary biomarker development. The Journal of Biological Chemistry, 288(37), 2688–2697.
Mendt, M., et al. (2018). Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight, 3(8).
Holland, E. C. (2000). Glioblastoma multiforme: The terminator. Proceedings of the National Academy of Sciences of the United States of America, 97(12), 6242–6244.
Graner, M. W., Cumming, R. I., & Bigner, D. D. (2007). The heat shock response and chaperones/heat shock proteins in brain tumors: Surface expression, release, and possible immune consequences. The Journal of Neuroscience, 27(42), 11214–11227.
Kore, R. A., & Abraham, E. C. (2014). Inflammatory cytokines, interleukin-1 beta and tumor necrosis factor-alpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochemical and Biophysical Research Communications, 453(3), 326–331.
Challagundla, K. B., et al. (2015). Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. Journal of the National Cancer Institute, 107(7), 1–13.
Marimpietri, D., et al. (2013). Proteome Profiling of Neuroblastoma-Derived Exosomes Reveal the Expression of Proteins Potentially Involved in Tumor Progression. PLoS One, 8(9).
Fong, M. Y., Zhou, W., Liu, L., Alontaga, A. Y., Chandra, M., Ashby, J., Chow, A., O’Connor, S. T. F., Li, S., Chin, A. R., Somlo, G., Palomares, M., Li, Z., Tremblay, J. R., Tsuyada, A., Sun, G., Reid, M. A., Wu, X., Swiderski, P., Ren, X., Shi, Y., Kong, M., Zhong, W., Chen, Y., & Wang, S. E. (2015). Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nature Cell Biology, 17(2), 183–194.
Tominaga, N., et al. (2015). Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nature Communications, 6.
Acknowledgements
Irit Sagi is an Incumbent of the Maurizio Pontecorvo Professorial Chair and wants to acknowledge European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 695437), Israel Science Foundation (1800/19), the USA-Israel Binational Science Foundation (712506-01) and The Rising Tide Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, A., Mohan, V., Krishnaswamy, V.R. et al. Exosomes as a storehouse of tissue remodeling proteases and mediators of cancer progression. Cancer Metastasis Rev 38, 455–468 (2019). https://doi.org/10.1007/s10555-019-09813-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-019-09813-5