Abstract
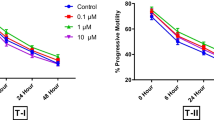
Human sperm banking is an important procedure in the assisted reproductive technique centers. It entails sperm damage. The aim of this study was to investigate beneficial effect of Ceratonia siliqua (C. siliqua) supplement in freezing/thawing media on post thaw sperm parameters and sperm chromatin quality in normozoospermic samples. Forty normozoospermic specimens were included in this prospective study. Each sample was divided into ten groups. In groups one to five, 0 (as control group) 5, 10, 20 and 30 µg/ml C. siliqua were added to freezing medium and in groups six to ten, similar concentration of C. siliqua were added to thawing medium for 30 min incubation. Sperm concentration, progressive motility, normal morphology, viability, aniline blue (AB), toluidine blue (TB) and sperm chromatin dispersion (SCD) staining tests were evaluated before vitrification and after thawing. The results showed that 10 and 20 µg/ml supplementation of C. siliqua in freezing/thawing media significantly increased progressive motility, normal morphology and viability of sperm (p < 0.05) as well as decreased AB, TB and SCD (p < 0.05). Also, 20 µg/ml had significantly higher improvement compared to 10 µg/ml C. siliqua (p < 0.05). The present study showed that C. siliqua supplemented freezing/thawing media can improve sperm quality of normozoospermic samples after freezing/thawing.
Similar content being viewed by others
Abbreviations
- ART:
-
Assisted reproductive technique
- C. siliqua :
-
Ceratonia siliqua
- AB:
-
Aniline blue
- TB:
-
Toluidine blue
- SCD:
-
Sperm chromatin dispersion
- ROS:
-
Reactive oxygen species
References
Agarwal A, Prabakaran SA et al (2005) Prevention of oxidative stress injury to sperm. J Androl 26(6):654–660
Agha-Rahimi A, Khalili MA et al (2014) Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod Biomed Online 28(3):352–358
Aghaz F, Khazaei M et al (2018) Cryoprotective effect of sericin supplementation in freezing and thawing media on the outcome of cryopreservation in human sperm. Aging Male 19:1–8
Alzoubi KH, Alibbini S et al (2018) Carob (Ceratonia siliqua L.) prevents short-term memory deficit induced by Chronic Stress in Rats. J Mol Neurosci 66(3):314–321
Amessis-Ouchemoukh N, Ouchemoukh S et al (2017) Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts. Ind Crops Prod 95:6–17
Ansari MS, Rakha BA et al (2011) Effect of straw size and thawing time on quality of cryopreserved buffalo (Bubalus bubalis) semen. Reprod Biol 11(1):49–54
Corsi L, Avallone R et al (2002) Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 73(7–8):674–684
Custódio L, Escapa AL et al (2011) Phytochemical profile, antioxidant and cytotoxic activities of the carob tree (Ceratonia siliqua L.) germ flour extracts. Plant Foods Hum Nutr 66(1):78–84
Di Santo M, Tarozzi N et al (2012) Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol 2012:854837. https://doi.org/10.1155/2012/854837
Donnelly ET, McClure N et al (2001) Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril 76(5):892–900
Forouzanfar M, Abid A et al (2013) Supplementation of sperm cryopreservation media with cell permeable superoxide dismutase mimetic agent (MnTE) improves goat blastocyst formation. Cryobiology 67(3):394–397
Franken D, Franken C et al (1999) Normal sperm morphology and chromatin packaging: comparison between aniline blue and chromomycin A3 staining. Andrologia 31(6):361–366
Gupta S, Agarwal A et al (2010) Recovery, preparation, storage and utilization of spermatozoa for fertility preservation in cancer patients and sub-fertile men. J Reprod Stem Cell Biotechnol 1(2):150–168
Hadi MY, Hameed IH et al (2017) Ceratonia siliqua: characterization, pharmaceutical products and analysis of bioactive compounds: a review. Res J Pharm Technol 10(10):3585–3589
Isachenko V, Maettner R et al (2011) Cryoprotectant-free vitrification of human spermatozoa in large (up to 0.5 mL) volume: a novel technology. Clinical laboratory 57(9–10):643–650
Johnston S, Satake N et al (2012) Osmotic stress and cryoinjury of koala sperm: an integrative study of the plasma membrane, chromatin stability and mitochondrial function. Reproduction 143(6):787–797
Kalthur G, Raj S et al (2011) Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw–induced DNA damage. Fertil Steril 95(3):1149–1151
Karimfar M, Niazvand F et al (2015) The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Inte J Immunopathol Pharmacol 28(1):69–76
Khalili MA, Adib M et al (2014) Vitrification of neat semen alters sperm parameters and DNA integrity. Urol J 11(2):1465–1470
Khazaei M, Pazhouhi M et al (2018) Evaluation of hydro-alcoholic extract of Trifolium Pratens L. for its anti-cancer potential on U87MG cell line. Cell J 20(3):412–421
Kopeika J, Thornhill A et al (2014) The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update 21(2):209–227
Mangoli E, Talebi AR et al (2018) Vitamin C attenuates negative effects of vitrification on sperm parameters, chromatin quality, apoptosis and acrosome reaction in neat and prepared normozoospermic samples. Taiwan J Obstet Gynecol 57(2):200–204
Meamar M, Zribi N et al (2012) Sperm DNA fragmentation induced by cryopreservation: new insights and effect of a natural extract from Opuntia ficus-indica. Fertil Steril 98(2):326–333
Meziani S, Oomah BD et al (2015) Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum. Microb Pathog 78:95–102
Nunez-Calonge R, Caballero P et al (2012) An improved experimental model for understanding the impact of sperm DNA fragmentation on human pregnancy following ICSI. Reprod Sci 19(11):1163–1168
Pavlovych O, Revenko O et al (2016) Optimization of thawing regimen for cryopreserved human sperm at normo-and pathospermia. Probl Cryobiol Cryomed 26(1):45–52
Rosenborg L, Rao K et al (1990) Changes in human sperm chromatin stability during preparation for in vitro fertilization. Int J Androl 13(4):287–296
Rossi T, Mazzilli F et al (2001) Improved human sperm recovery using superoxide dismutase and catalase supplementation in semen cryopreservation procedure. Cell Tissue Bank 2(1):9–13
Rtibi K, Jabri MA et al (2015) Gastroprotective effect of carob (Ceratonia siliqua L.) against ethanol-induced oxidative stress in rat. BMC Complement Alternat Med 15(1):292
Rtibi K, Selmi S et al (2017) Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J Sci Food Agric 97(8):2664–2670
Souli A, Sebai H et al (2015) Hepatoprotective effect of carob against acute ethanol-induced oxidative stress in rat. Toxicol Ind Health 31(9):802–810
Tarin J, Brines J et al (1998) Antioxidants may protect against infertility. Hum Reprod (Oxford, England) 13(6):1415–1416
Thomson LK, Fleming SD et al (2009) Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 24(9):2061–2070
Vafaei A, Mohammadi S et al (2018) Effects of carob (Ceratonia siliqua) on sperm quality, testicular structure, testosterone level and oxidative stress in busulfan-induced infertile mice. Pharm Sci 24(2):104–111
Viloria T, Garrido N et al (2007) Sperm selection by swim-up in terms of deoxyribonucleic acid fragmentation as measured by the sperm chromatin dispersion test is altered in heavy smokers. Fertil Steril 88(2):523–525
World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen
Acknowledgment
The authors are grateful to the staff at Motazedi Infertility Center for cooperating with us in the current study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Our institute ethics committee approved all procedures (IR.KUMS.REC.1397.259) and informed consents were obtained from individual patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faramarzi, A., Aghaz, F., Golestan Jahromi, M. et al. Does supplementation of sperm freezing/thawing media with Ceratonia siliqua improve detrimental effect of cryopreservation on sperm parameters and chromatin quality in normozoospermic specimens?. Cell Tissue Bank 20, 403–409 (2019). https://doi.org/10.1007/s10561-019-09779-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-019-09779-2