Abstract
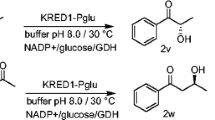
We have developed biocatalytic methods for the stereoselective reduction of cyclic prochiral 1,3-diketones for the production of optically active β-hydroxyketones and/or 1,3-diols. The recombinant ketoreductase KRED1-Pglu (formulated as purified catalyst) and whole cells of wild type Escherichia coli DE3 Star were used as biocatalysts, displaying different and sometimes complementary stereoselectivity, thus allowing the preparation of stereochemically pure β-hydroxyketones (12–66% isolated yields, > 99% e.e.) and 1,3-diols (40–60% isolated yields, > 99% e.e.).
Graphic Abstract





Similar content being viewed by others
References
Nakamura K, Matsuda T (2006) Curr Org Chem 10:1217–1246
Hoyos P, Sinisterra JV, Molinari F, Alcantara AR, Dominguez de Maria P (2010) Acc Chem Res 43:288–299
Sehl T, Hailes HC, Ward JM, Menyes U, Pohl M, Rother D (2014) Green Chem 16:3341–3348
Chen Y, Chen C, Wu X (2012) Chem Soc Rev 41:1742–1753
Kalaitzakis D, Rozzell JD, Smonou I, Kambourakis S (2006) Adv Synth Catal 348:1958–1969
Kalaitzakis D, Smonou I (2010) J Org Chem 75:8658–8661
Bataille CJR, Donohoe TJ (2011) Chem Soc Rev 40:114–128
Nguyen TN, Chen PA, Setthakarn K, May JA (2018) Molecules 23:2317
Paterson I, Chen DYK, Acena JL, Franklin AS (2000) Org Lett 2:1513–1516
Mukai K, Urabe D, Kasura S, Aoki N, Inoue MA (2013) Angew Chem Int Ed 52:5300–5304
Urabe D, Nakagawa Y, Mukai K, Fukushima K, Aoki N, Itoh H, Nagatomo M, Inoue M (2018) J Org Chem 83:13888–13910
Haberland J, Kriegesmann A, Wolfram E, Hummel W, Liese A (2002) Appl Microbiol Biotechnol 58:595–599
Grau BT, Devine PN, Di Michele LN, Kosjek B (2007) Org Lett 9:4951–4954
Lüdeke S, Richter M, Müller M (2009) Adv Synth Catal 351:253–259
Kurina-Sanz M, Bisogno FR, Lavandera I, Orden AA, Gotor V (2009) Adv Synth Catal 351:1842–1848
Husain SH, Stillger T, Dünkelmann P, Lödige M, Walter L, Breitling E, Pohl M, Bürchner M, Krossing I, Müller M, Romano D, Molinari F (2011) Adv Synth Catal 353:2359–2362
Mourelle-Insua A, de Gonzalo G, Lavandera I, Gotor-Fernández V (2018) Catalysts 8:150
Brooks W, Mazdiyasni M, Grothaus PG (1987) J Org Chem 52:3223–3232
Watanabe W, Iwamoto M, Nakada M (2005) J Org Chem 70:4652–4658
Kosmol H, Kieslich K, Vossing R, Koch HJ, Petzoldt K, Gibian H (1967) Justus Liebigs Ann Chem 701:199–205
Contente ML, Molinari F, Serra I, Pinto A, Romano D (2016) Eur J Org Chem 2016:1260–1263
Contente ML, Serra I, Brambilla M, Eberini I, Gianazza E, De Vitis V, Molinari F, Zambelli P, Romano D (2016) Appl Microbiol Biotechnol 100:193–201
Contente ML, Serra I, Molinari F, Gandolfi R, Pinto A, Romano D (2016) Tetrahedron 72:3974–3979
Contente ML, Molinari F, Zambelli P, De Vitis V, Gandolfi R, Pinto A, Romano D (2014) Tetrahedron Lett 55:7051–7053
Contente ML, Zambelli P, Galafassi S, Tamborini L, Pinto A, Conti P, Molinari F, Romano D (2015) J Mol Catal B 114:7–12
Contente ML, Serra I, Palazzolo L, Parravicini C, Gianazza E, Eberini I, Pinto A, Molinari F, Guidi B, Romano D (2016) Org Biomol Chem 14:3404–3408
Dall’Oglio F, Contente ML, Conti P, Molinari F, Monfredi D, Pinto A, Romano D, Ubiali D, Tamborini L (2017) Catal Commun 93:29–32
Manna MS, Mukherjee S (2014) Chem Sci 5:1627–1633
Byrne SJ, Fletcher AJ, Hebeisen P, Willis MC (2010) Org Biomol Chem 8:758–760
Oeggl R, Neumann T, Gätgens J, Romano D, Noack S, Rother D (2018) Front Bioeng Biotechnol 6:196
Yeung YY, Chein RJ, Corey EJ (2007) J Am Chem Soc 129:10346–10347
Kolakowski RV, Manpadi M, Zhang Y, Emge TJ, Williams LJ (2009) J Am Chem Soc 131:12910–12911
Salter GJ, Kell DB (1995) Crit Rev Biotechnol 15:139–177
Acknowledgements
The authors thank the University of Milan for funding the stay of Federica Dall’Oglio at the Biotechnology Forschungszentrum of Jülich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Contente, M.L., Dall’Oglio, F., Annunziata, F. et al. Stereoselective Reduction of Prochiral Cyclic 1,3-Diketones Using Different Biocatalysts. Catal Lett 150, 1176–1185 (2020). https://doi.org/10.1007/s10562-019-03015-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-03015-y