Abstract
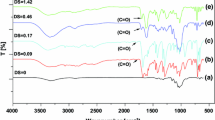
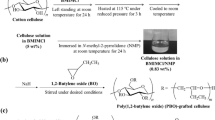
The cellulose solvent dimethylsulfoxide/tetrabutylammonium fluoride trihydrate (TBAF·3 H2O) was studied as reaction medium for the synthesis of benzyl cellulose (BC) by treating the dissolved polymer with benzyl chloride in the presence of solid NaOH or aqueous NaOH solution. BC samples with degree of substitution (DS) between 0.40 and 2.85 were accessible applying different molar ratios. The studies show that both the TBAF·3 H2O concentration and the molar ratio of the reagents to repeating unit influence the DS. The solubility of the BC synthesized in a different way, however, of comparable DS is different. Structural analyses were carried out by means of FTIR-, 1H- and 13C NMR spectroscopy. SEC measurements revealed polymer aggregation in samples of low DS synthesized in a solvent containing 9.0% TBAF·3 H2O. At higher concentration of TBAF·3 H2O in the solvent, the BC samples obtained do not form aggregates. BC of high DS is crystalline and shows thermotropic liquid crystalline behavior as analyzed by means of DSC. Melting point and degradation temperature are not related to the DS.
Similar content being viewed by others
References
Ass B.A.P., Frollini E. (2001). Aggregation of cellulose during dissolution and acetylation in N,N-dimethylacetamide/lithium chloride: an introductory study. Anais Assoc. Bras. Quim. 50(2):76–82
Ass B.A.P., Frollini E., Heinze Th. (2004). Studies on the homogeneous acetylation of linters cellulose in the novel solvent system tetrabutylammonium fluoride trihydrate/dimethylsulfoxide. Macromol. Biosci. 4:1008–1013
Awadel-Karim S., Nazhad M.M., Paszner L. (1999). Factors affecting crystalline structure of cellulose during solvent purification treatment. Holzforschung 53:1–8
Braun D., Meuret B. (1989). Benzylcellulose als thermoplatischer Kunststoff. I. Herstellung und Charakterisierung. Papier 43(12):688–694
Browning B.L. (1967). Methods of wood chemistry. John Wiley, New York, Vol. 2, pp. 882
Buschle-Diller G., Zeronian S.H. (1992). Enhancing the reactivity and strength of cotton fibres. J. Appl. Polym. Sci. 45:967–979
Ciacco G.T., Ass B.A.P., Ramos L.A., Frollini E. (2000). Acetylation of cellulose under homogeneous reaction conditions. In: Mattoso L.H.C., Leão A., Frollini E. (eds). Natural polymers and composites. EMBRAPA-USP-UNESP, Sao Paulo, Brazil, pp. 139–145
Ciacco G.T., Liebert T.F., Frollini E., Heinze Th. (2003). Application of the solvent dimethylsulfoxide/tetrabutylammonium fluoride trihydrate as reaction medium for the homogeneous acylation of sisal cellulose. Cellulose 10:125–132
Ciacco G.T., Ass B.A.P., Ramos L.A., Frollini E. (2004). Influence of the composition of the solvent system N,N-dimethylacetamide/lithium chloride on the solubility and acetylation of the sisal, sugarcane bagasse and Avicel celluloses. In: Mei L.H.C., Mattoso L.H.C., Curvelo A.A.S. (eds) Natural polymers and composites. EMBRAPA-USP- UNESP, Sao Paulo, Brazil, pp. 32–35
Da Roz A.L., Curvelo A.L. (2004). Thermal characterization of benzylcellulose derivatives prepared from bleached pinus Kraft pulp. J. Thermal Anal. 75:429–436
Heinze Th., Dicke R., Koschella A., Kull A.H. and Koch W. (2000) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol. Chem. Phys. 201:627–631
Ikeda I., Washino K. and Maeda Y. (2003) Graft polymerization of cyclic compounds on cellulose dissolved in tetrabutylammonium fluoride / dimethylsulfoxide. Sen’I Gakkaishi 59(3):110–114
Isogai A., Ishizu A. and Nakano J. (1984a). Preparation of tri-O-benzylcellulose by the use of nonaqueous cellulose solvents. J. Appl. Polym. Sci. 29: 2097–2109
Isogai A., Ishizu A. and Nakano J. (1984b). Preparation of tri-O-substituted cellulose ethers by the use of nonaqueous cellulose solvents. J. Appl. Polym. Sci. 29: 3873–3882
Isogai A., Ishizu A. and Nakano J. (1985). Thermal and structural properties of tri-O-substituted cellulose ethers. J. Appl. Polym. Sci. 30: 345–353
Isogai A., Ishizu A. and Nakano J. (1986). Preparation of tri-O-alkylcelluloses by the use of nonaqueous cellulose solvent and their physical characteristics. J. Appl. Polym. Sci. 31: 341–352
Just E.K. and Majewicz T.G. (1985). Cellulose Ethers. In: Mark H.F., Bikales N.M., Overberger C.G., Menger G. and Kroschwitz J.I. (eds) Encyclopedia of Polymer Science and Engineering. John Wiley and Sons. New York, Vol. 3, pp. 226–269
Liebert T. and Heinze Th. (1998). Synthesis path versus distribution of functional groups in cellulose ethers. Macromol. Symp. 130: 271–283
Liebert T.F. and Heinze Th. (2001). Exploitation of reactivity and selectivity in cellulose functionalization using unconventional media for the design of products showing new superstructures. Biomacromolecules 2: 1124–1132
Liebert T.F. and Heinze Th. (2005). Tailored Cellulose Esters: Synthesis and Structure Determination. Biomacromolecules 6(1): 333–340
McCormick CL, Lichatowich DK (1979) Homogeneous solution reactions of cellulose, chitin, and other polysaccharides to produce controlled-activity pesticide systems. J. Polym. Sci, Polym. Lett. Ed. 17: 479–484
Marson GA, El Seoud OA (1999) A novel, efficient procedure for acylation of cellulose under homogeneous solution conditions. J. Appl. Polym. Sci. 74: 1355–1360
Ramos L.A., Ciacco G.T., Assaf J.M., EL Seoud O.A. and Frollini E. (2002) Studies on dissolution and acetylation of microcrystalline, sisal, and cotton linters celluloses in DMAc/LiCl solvent system. In: Mattoso L.H.C., Frollini E. and Leão A (eds) Natural Polymers and Composites IV. EMBRAPA-USP- UNESP, Sao Paulo, Brazil, pp. 42–50
Takaragi A., Minoda M., Miyamoto T., Liu H.Q. and Zhang L.N. (1999). Reaction characteristics of cellulose in the LiCl/1,3-dimethyl-2-imidazolidinone solvent system. Cellulose 6: 93-102
TAPPI Press – Viscosity of pulp (capillary viscosimeter method) (1990). In: TAPPI Test Methods 1991. Tappi Press, Atlanta, 1, Ref. T230 om–89.
Acknowledgements
E. Frollini is grateful to FAPESP (The State of São Paulo Research Foundation, Brazil) for financial support and research fellowship for L. A. Ramos and CNPq (National Council of Research, Brazil) for research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramos, L.A., Frollini, E., Koschella, A. et al. Benzylation of cellulose in the solvent dimethylsulfoxide/tetrabutylammonium fluoride trihydrate. Cellulose 12, 607–619 (2005). https://doi.org/10.1007/s10570-005-9007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-005-9007-2