Abstract
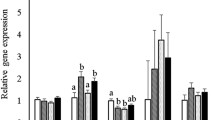
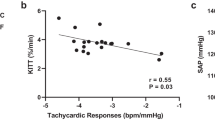
Although the deleterious influence of protein deficiency on fetal programming is well documented, the impact of a Western diet on epigenetic mechanisms is less clear. We hypothesized that high-fat high-sucrose diet (HFHSD) consumption during pregnancy leads to epigenetic modifications within the progeny’s compensatory renin–angiotensin system (RAS), affecting autonomic and metabolic functions. Dams were fed HFHSD (45% fat and 30% sucrose) or regular chow (RD) from mating until weaning of the pups (~7 weeks). Offspring from both groups were then maintained on chow and studied in adulthood (3–7 months). Offspring from HFHSD-exposed dams (OH) exhibited no difference in body weight or fasting blood glucose compared to controls (OR). In 3-month-old offspring, DNA methylation was significantly lower for the ACE2 gene (P < 0.05) in the brainstem, kidney and cecum. Moreover, ACE2 activity in the hypothalamus was increased at 7 months (OH: 91 ± 1 vs. OR: 74 ± 4 AFU/mg/min, P < 0.05). Although baseline blood pressure was not different between groups, vagal tone in OH was significantly impaired compared to OR. At the same time, OH offspring had a 1.7-fold increase in AT1a receptor expression and a 1.3-fold increase in ADAM17 mRNA. DOCA-salt treatment further revealed and exacerbated hypertensive response in the OH progeny (OH: 130 ± 6 vs. OR: 108 ± 3 mmHg, P < 0.05). Taken together, our data suggest that perinatal exposure to HFHSD resulted in epigenetic modifications of the compensatory brain RAS, potentially affecting plasticity of neuronal networks leading to autonomic dysfunction in the male offspring.



Similar content being viewed by others
References
Armitage JA, Lakasing L, Taylor PD, Balachandran AA, Jensen RI, Dekou V, Ashton N, Nyengaard JR, Poston L (2005) Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol 565(Pt 1):171–184. doi:10.1113/jphysiol.2005.084947
Barker DJ (1990) The fetal and infant origins of adult disease. BMJ: Brit Med J 301(6761):1111–1111
Barker DJ (1995) Fetal origins of coronary heart disease. BMJ 311(6998):171–174
Berghänel A, Heistermann M, Schülke O, Ostner J (2016) Prenatal stress effects in a wild, long-lived primate: predictive adaptive responses in an unpredictable environment. Proc Biol Sci 283(1839). doi:10.1098/rspb.2016.1304
Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E (2010) Angiotensin-I Converting Enzyme Type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes 59(10):2540–2548. doi:10.2337/db09-0782
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16(1):6–21. doi:10.1101/gad.947102
Boustany CM, Brown DR, Randall DC, Cassis LA (2005) AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 289(1):R181–R186. doi:10.1152/ajpregu.00507.2004
Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA (2007) Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 97(3):435–439. doi:10.1017/S0007114507352392
Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI (2005) Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 26(4):369–375
Chhabra KH, Chodavarapu H, Lazartigues E (2013) Angiotensin converting enzyme 2: a new important player in the regulation of glycemia. IUBMB Life 65(9):731–738. doi:10.1002/iub.1190
Chodavarapu H, Chhabra KH, Xia H, Shenoy V, Yue X, Lazartigues E (2016) High-fat diet-induced glucose dysregulation is independent of changes in islet ACE2 in mice. Am J Physiol - Regul Integr Comp Physiol 311(6):R1223–R1233. doi:10.1152/ajpregu.00362.2016
Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK (2008) Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension 51(3):712–718. doi:10.1161/hypertensionaha.107.100693
Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM (2014) Angiotensin II mediates angiotensin converting enzyme Type 2 internalization and degradation through an Angiotensin II Type I receptor-dependent mechanism. Hypertension 64(6):1368–1375. doi:10.1161/hypertensionaha.114.03743
Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MFR, Yuan L, Silver X, Torres R, Raizada MK (2006) ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 27(1):12–19
Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E (2008) Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin ii-mediated pressor and drinking responses and is associated with Angiotensin II Type 1 receptor downregulation. Circ Res 102(6):729–736. doi:10.1161/circresaha.107.169110
Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RAS, Speth RC, Sigmund CD, Lazartigues E (2010) Brain-selective overexpression of human angiotensin-converting enzyme Type 2 attenuates neurogenic hypertension. Circ Res 106(2):373–382. doi:10.1161/circresaha.109.208645
Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP (1997) Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Investig 100(9):2158–2169. doi:10.1172/JCI119752
Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA (2007) Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci USA 104(31):12796–12800. doi:10.1073/pnas.0705667104
Gomez-Guzman M, Toral M, Romero M, Jimenez R, Galindo P, Sanchez M, Zarzuelo MJ, Olivares M, Galvez J, Duarte J (2015) Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res 59(11):2326–2336. doi:10.1002/mnfr.201500290
Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA (2008) ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 295(3):R781–R788. doi:10.1152/ajpregu.00183.2008
Henriksen EJ (2007) Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 293(3):R974–R980. doi:10.1152/ajpregu.00147.2007
Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK (2005) Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol 90(5):783–790
Jin B, Li Y, Robertson KD (2011) DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer 2(6):607–617. doi:10.1177/1947601910393957
Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L (2003) Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41(1):168–175. doi:10.1161/01.hyp.0000047511.97879.fc
Khan I, Dekou V, Hanson M, Poston L, Taylor P (2004) Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 110(9):1097–1102. doi:10.1161/01.cir.0000139843.05436.a0
Ligthart S, Marzi C, Aslibekyan S, Mendelson MM, Conneely KN, Tanaka T, Colicino E, Waite LL, Joehanes R, Guan W, Brody JA, Elks C, Marioni R, Jhun MA, Agha G, Bressler J, Ward-Caviness CK, Chen BH, Huan T, Bakulski K, Salfati EL, Fiorito G, Wahl S, Schramm K, Sha J, Hernandez DG, Just AC, Smith JA, Sotoodehnia N, Pilling LC, Pankow JS, Tsao PS, Liu C, Zhao W, Guarrera S, Michopoulos VJ, Smith AK, Peters MJ, Melzer D, Vokonas P, Fornage M, Prokisch H, Bis JC, Chu AY, Herder C, Grallert H, Yao C, Shah S, McRae AF, Lin H, Horvath S, Fallin D, Hofman A, Wareham NJ, Wiggins KL, Feinberg AP, Starr JM, Visscher PM, Murabito JM, Kardia SLR, Absher DM, Binder EB, Singleton AB, Bandinelli S, Peters A, Waldenberger M, Matullo G, Schwartz JD, Demerath EW, Uitterlinden AG, van Meurs JBJ, Franco OH, Chen Y-DI, Levy D, Turner ST, Deary IJ, Ressler KJ, Dupuis J, Ferrucci L, Ong KK, Assimes TL, Boerwinkle E, Koenig W, Arnett DK, Baccarelli AA, Benjamin EJ, Dehghan A (2016) DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol 17(1):255. doi:10.1186/s13059-016-1119-5
Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC (2007) Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Brit J Nutr 97(6):1064–1073. doi:10.1017/s000711450769196x
Meaney MJ, Szyf M (2005) Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci 28(9):456–463. doi:10.1016/j.tins.2005.07.006
Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B (2015) Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genom 47(6):187–197. doi:10.1152/physiolgenomics.00136.2014
Mendoza A, Lazartigues E (2015) The compensatory renin-angiotensin system in the central regulation of arterial pressure: new avenues and new challenges. Therapeutic Adv Cardiovasc Disease 9(4):201–208. doi:10.1177/1753944715578056
O’Hare JD, Zsombok A (2016) Brain-liver connections: role of the preautonomic PVN neurons. Am J Physiol Endocrinol Metab 310(3):E183–E189. doi:10.1152/ajpendo.00302.2015
Patten GS, Adams MJ, Dallimore JA, Abeywardena MY (2004) Depressed prostanoid-induced contractility of the gut in spontaneously hypertensive rats (SHR) is not affected by the level of dietary fat. J Nutr 134(11):2924–2929
Pedersen KB, Sriramula S, Chhabra K, Xia H, Lazartigues E (2011) Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am J Physiol - Regul Integr Comp Physiol 301(5):1293–1299
Pedersen KB, Chodavarapu H, Porretta C, Robinson LK, Lazartigues E (2015) Dynamics of ADAM17-mediated shedding of ACE2 applied to pancreatic islets of male db/db mice. Endocrinology 156(12):4411–4425.
Reik W, Dean W, Walter J (2001) Epigenetic reprogramming in mammalian development. Science 293(5532):1089–1093. doi:10.1126/science.1063443
Sriramula S, Cardinale JP, Lazartigues E, Francis J (2011) ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res 92(3):401–408. doi:10.1093/cvr/cvr242
Sriramula S, Xia H, Xu P, Lazartigues E (2015) Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension 65(3):577–586. doi:10.1161/hypertensionaha.114.04691
Sriramula S, Pedersen KB, Xia H, Lazartigues E (2017) Determining the enzymatic activity of angiotensin-converting enzyme 2 (ACE2) in brain tissue and cerebrospinal fluid using a quenched fluorescent substrate. Methods Mol Biol 1527:117–126
Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK (2006) Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol - Heart Circ Physiol 291(1):H482–H483
Stein CE, Fall CHD, Kumaran K, Osmond C, Barker DJP, Cox V (1996) Fetal growth and coronary heart disease in South India. Lancet 348(9037):1269–1273. doi:10.1016/S0140-6736(96)04547-3
Tain Y-L, Lee W-C, Wu KLH, Leu S, Chan JYH (2016) Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J Nutr Biochem 38:86–92. doi:10.1016/j.jnutbio.2016.08.006
Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, Srinivasan K (2015) Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol 93(3):343–351. doi:10.1016/j.bcp.2014.11.013
Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, Tsai P-C, Ried JS, Zhang W, Yang Y, Tan S, Fiorito G, Franke L, Guarrera S, Kasela S, Kriebel J, Richmond RC, Adamo M, Afzal U, Ala-Korpela M, Albetti B, Ammerpohl O, Apperley JF, Beekman M, Bertazzi PA, Black SL, Blancher C, Bonder M-J, Brosch M, Carstensen-Kirberg M, de Craen AJM, de Lusignan S, Dehghan A, Elkalaawy M, Fischer K, Franco OH, Gaunt TR, Hampe J, Hashemi M, Isaacs A, Jenkinson A, Jha S, Kato N, Krogh V, Laffan M, Meisinger C, Meitinger T, Mok ZY, Motta V, Ng HK, Nikolakopoulou Z, Nteliopoulos G, Panico S, Pervjakova N, Prokisch H, Rathmann W, Roden M, Rota F, Rozario MA, Sandling JK, Schafmayer C, Schramm K, Siebert R, Slagboom PE, Soininen P, Stolk L, Strauch K, Tai ES, Tarantini L, Thorand B, Tigchelaar EF, Tumino R, Uitterlinden AG, van Duijn C, van Meurs JBJ, Vineis P, Wickremasinghe AR, Wijmenga C, Yang T-P, Yuan W, Zhernakova A, Batterham RL, Smith GD, Deloukas P, Heijmans BT, Herder C, Hofman A, Lindgren CM, Milani L, van der Harst P, Peters A, Illig T, Relton CL, Waldenberger M, Järvelin M-R, Bollati V, Soong R, Spector TD, Scott J, McCarthy MI, Elliott P, Bell JT, Matullo G, Gieger C, Kooner JS, Grallert H, Chambers JC (2017) Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541(7635):81–86. doi:10.1038/nature20784
Wu L, Shi A, Zhu D, Bo L, Zhong Y, Wang J, Xu Z, Mao C (2016) High sucrose intake during gestation increases angiotensin II type 1 receptor-mediated vascular contractility associated with epigenetic alterations in aged offspring rats. Peptides 86:133–144. doi:10.1016/j.peptides.2016.11.002
Wulsin LR, Horn PS, Perry JL, Massaro JM, D’Agostino RB (2015) Autonomic Imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J Clin Endocrinol Metab 100(6):2443–2448. doi:10.1210/jc.2015-1748
Xia H, Lazartigues E (2010) Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep 12(3):170–175. doi:10.1007/s11906-010-0105-7
Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E (2009) Angiotensin II Type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53:210–216. doi:10.1161/hypertensionaha.108.123844
Xia H, Sriramula S, Chhabra K, Lazartigues E (2013) Brain ACE2 shedding contributes to the development of neurogenic hypertension. Circ Res 113:1087–1096
Xiao L, Gao L, Lazartigues E, Zucker IH (2011) Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension 58(6):1057–1065. doi:10.1161/hypertensionaha.111.176636
Xu P, Sriramula S, Lazartigues E (2011) ACE2/Ang-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol - Regul Integr Comp Physiol 300(4):R804–817. doi:10.1152/ajpregu.00222.2010
Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK (2007) Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49(4):926–931
Yamazato Y, Ferreira AJ, Hong K-H, Sriramula S, Francis J, Yamazato M, Yuan L, Bradford CN, Shenoy V, Oh SP, Katovich MJ, Raizada MK (2009) Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension 54(2):365–371. doi:10.1161/hypertensionaha.108.125468
Zelena D, Filaretova L, Mergl Z, Barna I, Tóth ZE, Makara GB (2006) Hypothalamic paraventricular nucleus, but not vasopressin, participates in chronic hyperactivity of the HPA axis in diabetic rats. Am J Physiol Endocrinol Metab 290(2):E243–E250. doi:10.1152/ajpendo.00118.2005
Zheng H, Liu X, Patel KP (2011) Angiotensin-converting enzyme 2 overexpression improves central nitric oxide mediated sympathetic outflow in chronic heart failure. Am J Physiol Heart Circ Physiol. doi:10.1152/ajpheart.00330.2011
Acknowledgements
The authors would like to thank Dr. Weining Tang (Omega Bio-tek, Norcross, GA) for providing technical expertise regarding the design of the methylation array.
Funding
This work was supported by an Established Investigator Award from the American Heart Association (12EIA8030004 to E.L.), the National Center for Research Resources (RR018766), and the National Institute of General Medical Sciences (GM103514 and GM106392).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Mukerjee, S., Zhu, Y., Zsombok, A. et al. Perinatal Exposure to Western Diet Programs Autonomic Dysfunction in the Male Offspring. Cell Mol Neurobiol 38, 233–242 (2018). https://doi.org/10.1007/s10571-017-0502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0502-4