Abstract
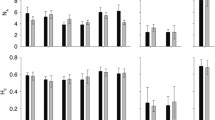
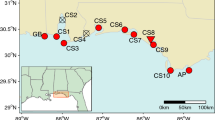
Recognising patterns of genetic diversity and connectivity is integral to understanding the mechanisms behind population declines and formulating management plans for the conservation of threatened or endangered species. This is particularly important for clonal organisms such as seagrasses, which are experiencing rapid global decline. This study quantifies genetic diversity within 12 naturally fragmented Posidonia australis meadows on the east coast of Australia, using a set of eight microsatellite DNA markers. Genetic diversity increased with latitude, moving away from the range-edge, and was significantly lower in six mid-range endangered meadows and the two northernmost meadows. These meadows also showed evidence of shared multilocus genotypes despite significant geographic separation. The four southernmost meadows were genetically differentiated from all other meadows further north, and all multilocus genotypes identified were unique to their sample locations. We conclude that patterns of low diversity in the endangered and northern meadows are likely due to a population bottleneck caused by a range-edge effect. A common ancestral source meadow existing prior to historical sea level changes may explain the sharing of multilocus genotypes, as contemporary gene flow between these geographically isolated meadows is unlikely. Our findings have important implications for conservation, highlighting the endangered and range-edge populations as those potentially most at risk of extinction should environmental conditions change. These results can be utilised for the location of suitable donor populations for transplanting purposes as a means of mitigating further declines.





Similar content being viewed by others
References
Aires T, Marbà N, Cunha RL, Kendrick GA, Walker DI, Serrão EA, Duarte CM, Arnaud-Haond S (2011) Evolutionary history of the seagrass genus Posidonia. Mar Ecol Prog Ser 421:117–130
Arnaud-Haond S, Belkhir K (2007) GENCLONE: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organisation. Mol Ecol Notes 7:15–17
Arnaud-Haond S, Teixeira S, Massa SI, Billot C, Saenger P, Coupland G, Duarte CM, Serrão EA (2006) Genetic structure at range-edge: low diversity and high inbreeding in Southeast Asian mangrove (Avicennia marina) populations. Mol Ecol 15:3515–3525
Arnaud-Haond S, Duarte M, Alberto F, Serrão EA (2007a) Standardizing methods to address clonality in population studies. Mol Ecol 16:5115–5139
Arnaud-Haond S, Migliaccio M, Diaz-Almela E, Teixeira S, van de Vliet MS, Alberto F, Procaccini G, Duarte CM, Serrão EA (2007b) Vicariance patterns in the Mediterranean Sea: east–west cleavage and low dispersal in the endemic seagrass Posidonia oceanica. J Biogeogr 34:963–976
Arnaud-Haond S, Duarte CM, Diaz-Almela E, Marbà N, Sintes T, Serrão EA (2012) Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLoS One 7:e30454
Australian Government Department of Climate Change and Energy Efficiency (2009) Australia’s coast—its nature and importance. In: Climate change risks to Australia’s coast: a first pass national assessment. DCCEE, Canberra. http://www.climatechange.gov.au/publications/coastline/climate-change-risks-to-australias-coasts.aspx. Accessed 20 Dec 2012
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The valve of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193
Barrett SCH, Eckert CG, Husband BC (1993) Evolutionary processes in aquatic plant populations. Aquat Bot 44:105–145
Bastyan GR, Cambridge ML (2008) Transplantation as a method for restoring the seagrass Posidonia australis. Estuar Coast Shelf Sci 79:289–299
Beatty GE, McEvoy PM, Sweeney O, Provan J (2008) Range-edge effects promote clonal growth in peripheral populations of the one-sided wintergreen Orthilia secunda. Divers Distrib 14:546–555
Bohonak AJ, Davies N, Roderick GK, Villablanca FX (1998) Is population genetics mired in the past? Trends Ecol Evol 13:360
Bossart JL, Prowell DP (1998) Genetic estimates of population structure and gene flow: limitations, lessons and new directions. Trends Ecol Evol 13:202–206
Coleman MA, Chambers J, Knott NA, Malcolm HA, Harasti D, Jordan A, Kelaher BP (2011) Connectivity within and among a network of temperate marine reserves. PLoS One 6:e20168
Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Creese RG, Glasby TM, West G, Gallen C (2009) Mapping the habitats of NSW estuaries. Industry & Investment NSW Fisheries Final Report Series 113. Port Stephens. ISSN 1837-2112
Cunha AH, Marbá NN, van Katwijk MM, Pickerell C, Henriques M, Bernard G, Ferreira MA, Garcia S, Garmendia JM, Manent P (2012) Changing paradigms in seagrass restoration. Restor Ecol 20:427–430
De Meester L, Louette G, Duvivier C, Van Damme C, Michels E (2007) Genetic composition of resident populations influences establishment success of immigrant species. Oecologia 153:431–440
Diekmann OE, Serrão EA (2012) Range-edge genetic diversity: locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Mol Ecol 21:1647–1657
Duarte CM (2002) The future of seagrass meadows. Environ Conserv 29:192–206
Duffy JE (2006) Biodiversity and the functioning of seagrass ecosystems. Mar Ecol Prog Ser 311:233–250
Ellers J (2009) Evolutionary processes in community ecology. In: Verhoef HA, Morin PJ (eds) Community ecology: processes, models, and applications. Oxford University Press, Oxford, pp 151–162
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics analysis. Evol Bioinform Online 1:47–50
Fitzpatrick J, Kirkman H (1995) Effects of prolonged shading stress on growth and survival of seagrass Posidonia australis in Jervis Bay, New South Wales, Australia. Mar Ecol Prog Ser 127:279–289
Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krause-Jensen D, McGlathery KJ, Serrano O (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:505–509
Gillanders BM (2006) Seagrass, fish and fisheries. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, New York, pp 503–536
Gobert S, Cambridge ML, Velimirov B, Pergent G, Lepoint G, Bouquegneau J, Dauby P, Pergent-Martini C, Walker DI (2006) Biology of Posidonia. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, New York, pp 387–408
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
He T, Rao G, You R, Song G, Zhang D (2000) Genetic diversity of widespread Ophiopogon intermedius (Liliaceae s.l.): a comparison with endangered O. xylorrhizus. Biol Conserv 96:253–257
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AS, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108
Hughes AR, Stachowicz JJ (2004) Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc Natl Acad Sci USA 101:8998–9002
Hughes AR, Stachowicz JJ (2011) Seagrass genotypic diversity increases disturbance response via complementarity and dominance. J Ecol 99:445–453
Hughes AR, Williams SL, Duarte CM, Heck KL, Waycott M (2009) Associations of concern: declining seagrasses and threatened dependent species. Front Ecol Environ 7:242–246
Hughes AR, Best RJ, Stachowicz JJ (2010) Genotypic diversity and grazer identity interactively influence seagrass and grazer biomass. Mar Ecol Prog Ser 403:43–51
Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genet 6:13–18
Johannesson K, André C (2006) Life on the margin: genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Mol Ecol 15:2013–2029
Kendrick GA, Waycott M, Carruthers T, Cambridge M, Hovey R, Krauss SL, Lavery P, Les DH, Lowe R, Mascaró O, Ooi Lean Sim J, Orth RJ, Rivers D, Ruiz-Montoya L, Sinclair EA, Statton J, van Dijk K, Verduin J (2012) The central role of dispersal in the maintenance and persistence of seagrass populations. Bioscience 62:56–65
Kuo JJ (2011) Posidoniaceae. Flora Aust 39:111–120
Larkum AWD, West RJ (1990) Long-term changes of seagrass meadows in Botany Bay, Australia. Aquat Bot 37:55–70
Lowe WH, Allendorf FW (2010) What can genetics tell us about population connectivity? Mol Ecol 19:3038–3051
Mateo MA, Cebrián J, Dunton K, Mutchler T (2006) Carbon flux in seagrass ecosystems. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, New York, pp 159–192
McMahon K, Lavery PS, Mulligan M (2011) Recovery from the impact of light reduction on the seagrass Amphibolis griffithii, insights for dredging management. Mar Pollut Bull 62:270–283
Meehan AJ, West RJ (2000) Recovery times for a damaged Posidonia australis bed in south eastern Australia. Aquat Bot 67:161–167
Meehan AJ, West RJ (2002) Experimental transplanting of Posidonia australis seagrass in Port Hacking, Australia, to assess the feasibility of restoration. Mar Pollut Bull 44:25–31
Meehan AJ, West RJ (2004) Seedling development and patch formation of the seagrass Posidonia australis in a southeast Australian estuary. Aquat Bot 79:1–14
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Neigel JE (2002) Is FST obsolete? Conserv Genet 3:167–173
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Procaccini G, Piazzi L (2001) Genetic polymorphism and transplantation success in the Mediterranean seagrass Posidonia oceanica. Restor Ecol 9:332–338
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Reusch TBH (2001) New markers—old questions: population genetics of seagrasses. Mar Ecol Prog Ser 211:261–274
Reusch TBH, Hughes AR (2006) The emerging role of genetic diversity for ecosystem functioning: estuarine macrophytes as models. Estuar Coast 29:159–164
Reusch TBH, Ehlers A, Hämmerli A, Worm B (2005) Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc Natl Acad Sci USA 102:2826–2831
Reynolds LK, McGlathery KJ, Waycott M (2012) Genetic diversity enhances restoration success by augmenting ecosystem services. PLoS One 7:e38397
Rousset F (2008) Genepop’007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Res 8:103–106
Ruggiero MV, Turk R, Procaccini G (2002) Genetic identity and homozygosity in North-Adriatic populations of Posidonia oceanica: an ancient, post-glacial clone? Conserv Genet 3:71–74
Schmid B (1994) Effects of genetic diversity in experimental stands of Solidago altissima—evidence for the potential role of pathogens as selective agents in plant populations. J Ecol 82:165–175
Short FT, Polidoro B, Livingstone SR, Carpenter KE, Bandeira S, Bujang JS, Calumpong HP, Carruthers TJB, Coles RG, Dennison WC, Erftemeijer PLA, Fortes MD, Freeman AS, Jagtap TG, Kamal AHM, Kendrick GA, Kenworthy WJ, La Nafie YA, Nasution IM, Orth RJ, Prathep A, Sanciangco JC, van Tussenbroek B, Vergara SG, Waycott M, Zieman JC (2011) Extinction risk assessment of the world’s seagrass species. Biol Conserv 144:1961–1971
Sinclair EA, Anthony J, Coupland GT, Waycott M, Barrett MD, Barrett RL, Cambridge ML, Wallace MJ, Dixon KW, Krauss SL, Kendrick GA (2009) Characterisation of polymorphic microsatellite markers in the widespread Australian seagrass, Posidonia australis Hook. f. (Posidoniaceae), with cross amplification in the sympatric P. sinuosa. Conserv Genet Resour 1:273–276
Sinclair EA, Verduin J, Krauss SK, Hardinge J, Anthony J, Kendrick GA (2013) A genetic assessment of a successful seagrass meadow (Posidonia australis) restoration trial. Ecol Manag Restor 14(1):68–71
Stachowicz JJ, Bruno JF, Duffy JE (2007) Understanding the effects of marine biodiversity on communities and ecosystems. Annu Rev Ecol Evol Syst 38:739–766
Valière N (2002) GIMLET: a computer program for analysing genetic individual identification data. Mol Ecol Notes 2:377–379
van Andel J (1998) Intraspecific variability in the context of ecological restoration projects. Perspect Plant Ecol 1:221–227
van Katwijk MM, Bos AR, de Jonge VN, Hanssen LSAM, Hermus DCR, de Jong DJ (2009) Guidelines for seagrass restoration: importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Mar Pollut Bull 58:179–188
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Waycott M (1998) Genetic variation, its assessment and implications to the conservation of seagrasses. Mol Ecol 7:793–800
Waycott M, James SH, Walker DI (1997) Genetic variation within and between populations of Posidonia australis, a hydrophilous, clonal seagrass. Heredity 79:408–417
Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA 106:12377–12381
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
West RJ, Thorogood CA, Walford TR, Williams RJ (1985) Estuarine inventory for New South Wales, Australia. Fisheries Bulletin No. 2. NSW Department of Agriculture, Sydney
Williams SL (2001) Reduced genetic diversity in eelgrass transplantations affects both population growth and individual fitness. Ecol Appl 11(1472–14):8
Wolf AT, Howe RW, Hamrick JL (2000) Genetic diversity and population structure of the serpentine endemic Calystegia collina (Convolvulaceae) in Northern California. Am J Bot 87:1138–1146
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Acknowledgments
This research was undertaken with the support of an Australian Postgraduate Award at the University of New South Wales. The genotyping was undertaken at the Botanical Gardens and Parks Authority in Perth. Financial support was provided by an Australian Research Council Linkage Grant (LP0991150), with Industry Partners Cockburn Cement, Department of Environment and Conservation (now Department of Parks and Wildlife) Western Australia, and the Botanic Gardens and Parks Authority, Western Australia. We thank Melinda Coleman for advice on genetic analyses, Alexandra Campbell, Ezequiel Marzinelli, Mariana Mayer-Pinto and Ray Blick for constructive comments that improved this manuscript and the Evolution & Ecology Research Centre and the Centre for Marine Bio-Innovation for funding and support. All research was conducted with the permission of New South Wales Fisheries (Permit No. P11/0059-1.1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10592_2014_573_MOESM1_ESM.docx
Supplementary Table 1 Pairwise FST estimates between all locations are displayed below diagonal. Corresponding P-values are displayed above diagonal, withsites that are significantly genetically different from each other shown in bold. Shaded areas represent within status (endangered/non-endangered)pairwise comparisons. Location abbreviations are Wallis Lake (WL), Port Stephens (PS), Lake Macquarie (LM), Brisbane Water (BW), Pittwater(PW), Sydney Harbour (SH), Botany Bay (BB), Port Hacking (PH), Jervis Bay (JB), St. Georges Basin (SG), Merimbula Lake (ML) and PambulaLake (PL) (DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Evans, S.M., Sinclair, E.A., Poore, A.G.B. et al. Genetic diversity in threatened Posidonia australis seagrass meadows. Conserv Genet 15, 717–728 (2014). https://doi.org/10.1007/s10592-014-0573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-014-0573-4