Abstract
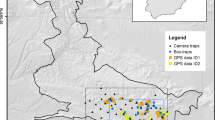
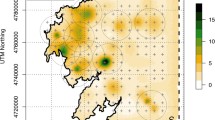
Populations of vertebrates are built of individuals of different sex, age class or stage, which often affect distinctly the population dynamics. Such intrapopulation partitioning of vital rates needs to be identified to develop efficient conservation actions. Using DNA extracted from feces and feathers we combined DNA-tagging and mark–recapture analyses to evaluate sex-specific population dynamics of an endangered population of capercaillie (Tetrao urogallus; Phasianidae). We built encounter histories for 120 individuals in the mating seasons of 2009–2011, in a study area of about 424 km2. Minimum number of individuals per mating season and estimates of population size ranged 56–67 and 76–115, respectively. Estimates of population size were consistently lower in multiple-season, open-population models than in single-season closed-population models. The super-population in the study area was 149 individuals for the whole study period. Sex-ratio was notably male-biased. Probability of recapture p ranged 0.62–0.70, and was similar for males and females. Female apparent survival φ was lower than expected, and much lower than male apparent survival. It includes however movements in and out of the sampled population, thus comparison with previously reported values based on conventional tagging should be cautious. Females showed higher turnover, indicated by higher probability β of entering the sampled population, and higher number of entries from the super-population, Bgross. Realized population growth rate ʎ was > 1 for both females and males. The combination of non-intrusive DNA-tagging and the analytical framework of mark–recapture models provided inferences on population dynamics that would have been hardly feasible with conventional methods. Male-biased sex ratios, higher female turnover and seemingly low female apparent survival were our key findings. While the whole population needs continuous monitoring, we believe that adult females deserve priority attention in evaluation and design of conservation actions.



Similar content being viewed by others
References
Anderson DR, Burnham KP, Thompson WL (2000) Null hypothesis testing: problems, prevalence, and an alternative. J Wildl Manag. https://doi.org/10.2307/3803199
Augustine BC, Kéry M, Marin JO, Mollet P, Pasinelli G, Sutherland C (2019) Sex-specific population dynamics and demography of capercaillie (Tetrao urogallus L.) in a patchy environment. bioRxiv. https://doi.org/10.1101/576876
Bañuelos MJ, Quevedo M (2008) Update of the situation of the Cantabrian capercaillie Tetrao urogallus cantabricus: an ongoing decline. Grouse News 35:5–7
Bañuelos MJ, Quevedo M, Obeso JR (2008) Habitat partitioning in endangered Cantabrian capercaillie Tetrao urogallus cantabricus. J Ornithol 149:245–252. https://doi.org/10.1007/s10336-007-0267-5
Bessa-Gomes C, Legendre S, Clobert J (2004) Allee effects, mating systems and the extinction risk in populations with two sexes. Ecol Lett 7:802–812
Biro PA, Dingemanse NJ (2009) Sampling bias resulting from animal personality. Trends Ecol Evol 24:66–67. https://doi.org/10.1016/j.tree.2008.11.001
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28. https://doi.org/10.1086/343878
Boyce MS (1992) Population viability analysis. Annu Rev Ecol Syst 23:481–506
Bradshaw CJ, Barker RJ, Harcourt RG, Davis LS (2003) Estimating survival and capture probability of fur seal pups using multistate mark–recapture models. J Mammal 84:65–80
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Caizergues A, Dubois S, Loiseau A, Mondor G, Rasplus JY (2001) Isolation and characterization of microsatellite loci in black grouse (Tetrao tetrix). Mol Ecol Notes 1:36–38
Carvalho SB, Torres J, Tarroso P, Velo-Antón G (2019) Genes on the edge: a framework to detect genetic diversity imperiled by climate change. Glob Chang Biol. https://doi.org/10.1111/gcb.14740
Castroviejo J, Delibes M, García-Dory MA, Garzón J (1974) Censo de urogallos cantabricos (Tetrao urogallus cantabricus). Asturnatura 2:53–74
Caswell H (2001) Matrix population models: construction, analysis, and interpretation. Sinauer Associates, Sunderland
Charlesworth B (2009) Effective population size and patterns of molecular evolution and variation. Nat Rev Genet 10:195–205. https://doi.org/10.1038/nrg2526
Cilimburg AB, Lindberg MS, Tewksbury JJ, Hejl SJ (2002) Effects of dispersal on survival probability of adult Yellow Warblers (Dendroica petechia). Auk 119:778–789
Cooch EG, White GC (eds) (2017) Program MARK—a gentle introduction, 17th edn.
Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cormack RM (1964) Estimates of survival from the sighting of marked animals. Biometrika 51:429. https://doi.org/10.2307/2334149
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Donald PF (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Durell S (2000) Individual feeding specialisation in shorebirds: population consequences and conservation implications. Biol Rev 75:503–518
Frankham R (1995) Effective population size/adult population size ratios in wildlife: a review. Genet Res 66:95–107. https://doi.org/10.1017/S0016672308009695
Frankham R, Bradshaw CJA, Brook BW (2014) Genetics in conservation management: revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol Conserv 170:56–63. https://doi.org/10.1016/j.biocon.2013.12.036
Galpern P, Manseau M, Hettinga P, Smith K, Wilson P (2012) Allelematch: an R package for identifying unique multilocus genotypes where genotyping error and missing data may be present. Mol Ecol Resour 12:771–778
Grimm V, Storch I (2000) Minimum viable population size of capercaillie Tetrao urogallus: results from a stochastic model. Wildl Biol 6:219–225
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467
Hannon SJ, Martin K (2006) Ecology of juvenile grouse during the transition to adulthood. J Zool 269:422–433
Hogan FE, Cooke R, Burridge CP, Norman JA (2008) Optimizing the use of shed feathers for genetic analysis. Mol Ecol Resour 8:561–567
Jacob G, Debrunner R, Gugerli F, Schmid B, Bollmann K (2010) Field surveys of capercaillie (Tetrao urogallus) in the Swiss Alps underestimated local abundance of the species as revealed by genetic analyses of non-invasive samples. Conserv Genet 11:33–44
Johnsgard PA (1983) The grouse of the world. Croom Helm, Lincoln
Johnson KH, Braun CE (1999) Viability and conservation of an exploited sage grouse population. Conserv Biol 13:77–84. https://doi.org/10.1046/j.1523-1739.1999.97284.x
Jolly GM (1965) Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrika 52:225–247
Katzner TE, Ivy JAR, Bragin EA, Milner-Gulland EJ, DeWoody JA (2011) Conservation implications of inaccurate estimation of cryptic population size. Anim Conserv 14:328–332. https://doi.org/10.1111/j.1469-1795.2011.00444.x
Laiolo P, Bañuelos MJ, Blanco-Fontao B, García M, Gutiérrez G (2011) Mechanisms underlying the bioindicator notion: spatial association between individual sexual performance and community diversity. PLoS ONE 6:e22724. https://doi.org/10.1371/journal.pone.0022724
Lambertucci SA, Carrete M, Speziale KL, Hiraldo F, Donázar JA (2013) Population sex ratios: another consideration in the reintroduction—reinforcement debate? PLoS ONE 8:e75821. https://doi.org/10.1371/journal.pone.0075821
Lande R (1988) Demographic models of the northern spotted owl (Strix occidentalis caurina). Oecologia 75:601–607
Legendre S, Clobert J, Møller AP, Sorci G (1999) Demographic stochasticity and social mating system in the process of extinction of small populations: the case of passerines introduced to new zealand. Am Nat 153:449–463. https://doi.org/10.1086/303195
Lindström J, Kokko H (1998) Sexual reproduction and population dynamics: the role of polygyny and demographic sex differences. Proc R Soc Lond B 265:483–488
Luikart G, Ryman N, Tallmon DA, Schwartz MK, Allendorf FW (2010) Estimation of census and effective population sizes: the increasing usefulness of DNA-based approaches. Conserv Genet 11:355–373. https://doi.org/10.1007/s10592-010-0050-7
Lukacs PM, Burnham KP (2005) Review of capture-recapture methods applicable to noninvasive genetic sampling. Mol Ecol 14:3909–3919
Margalida A, Oro D, Cortés-Avizanda A, Heredia R, Donázar JA (2011) Misleading population estimates: biases and consistency of visual surveys and matrix modelling in the endangered bearded vulture. PLoS ONE 6:e26784. https://doi.org/10.1371/journal.pone.0026784
Marucco F, Boitani L, Pletscher DH, Schwartz MK (2011) Bridging the gaps between non-invasive genetic sampling and population parameter estimation. Eur J Wildl Res 57:1–13. https://doi.org/10.1007/s10344-010-0477-7
Marucco F, Vucetich LM, Peterson RO, Adams JR, Vucetich JA (2012) Evaluating the efficacy of non-invasive genetic methods and estimating wolf survival during a ten-year period. Conserv Genet 13:1611–1622. https://doi.org/10.1007/s10592-012-0412-4
McCain C, Szewczyk T, Bracy Knight K (2016) Population variability complicates the accurate detection of climate change responses. Glob Chang Biol 22:2081–2093. https://doi.org/10.1111/gcb.13211
McDougall PT, Reale D, Sol D, Reader SM (2006) Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Anim Conserv 9:39–48
McKelvey KS, Schwartz MK (2005) Dropout: a program to identify problem loci and samples for noninvasive genetic samples in a capture-mark–recapture framework. Mol Ecol Notes 5:716–718
McNew LB, Gregory AJ, Wisely SM, Sandercock BK (2012) Demography of greater prairie-chickens: regional variation in vital rates, sensitivity values, and population dynamics. J Wildl Manag 76:987–1000. https://doi.org/10.1002/jwmg.369
Miller CR, Joyce P, Waits LP (2002) Assessing allelic dropout and genotype reliability using maximum likelihood. Genetics 160:357–366
Miller CR, Joyce P, Waits LP (2005) A new method for estimating the size of small populations from genetic mark–recapture data. Mol Ecol 14:1991–2005
Mills LS, Citta JJ, Lair KP, Schwartz MK, Tallmon DA (2000) Estimating animal abundance using noninvasive DNA sampling: promise and pitfalls. Ecol Appl 10:283–294
Mollet P, Kéry M, Gardner B, Pasinelli G, Royle JA (2015) Estimating population size for capercaillie (Tetrao urogallus L.) with spatial capture-recapture models based on genotypes from one field sample. PLoS ONE 10:e0129020. https://doi.org/10.1371/journal.pone.0129020
Morán-Luis M (2017) Individual movements, demography and viability of an endangered population: the Cantabrian Capercaillie. PhD Thesis. Universidad de Oviedo
Morán-Luis M, Fameli A, Blanco-Fontao B, Fernández-Gil A, Rodríguez-Muñoz R, Quevedo M, Mirol P, Bañuelos M-J (2014) Demographic status and genetic tagging of endangered capercaillie in NW Spain. PLoS ONE 9:e99799. https://doi.org/10.1371/journal.pone.0099799
Moss R (2001) Second extinction of capercaillie (Tetrao urogallus) in Scotland? Biol Conserv 101:255–257
Moss R, Oswald J (1985) Population dynamics of Capercaillie in a north-east Scottish glen. Ornis Scand 16:229–238
Moss R, Picozzi N, Summers RW, Baines D (2000) Capercaillie Tetrao urogallus in Scotland—demography of a declining population. Ibis 142:259–267
Moss R, Picozzi N, Catt DC (2006) Natal dispersal of capercaillie Tetrao urogallus in northeast Scotland. Wildl Biol 12:227–232
Mulders R, Boulanger J, Paetkau D (2007) Estimation of population size for wolverines Gulo gulo at Daring Lake, Northwest Territories, Using DNA based mark–recapture methods. WBIO 13:38–51
Palsbøll PJ (1999) Genetic tagging: contemporary molecular ecology. Biol J Linn Soc 68:3–22
Pennell MW, Stansbury CR, Waits LP, Miller CR (2013) CAPWIRE: a R package for estimating population census size from non-invasive genetic sampling. Mol Ecol Resour 13:154–157
Pérez T, Vázquez JF, Quirós F, Domínguez A (2011) Improving non-invasive genotyping in capercaillie (Tetrao urogallus): redesigning sexing and microsatellite primers to increase efficiency on faeces samples. Conserv Genet Resour 3:483–487
Piertney SB, Höglund J (2001) Polymorphic microsatellite DNA markers in black grouse (Tetrao tetrix). Mol Ecol Notes 1:303–304
Pollo C, Robles L, Seijas JM, García-Miranda A, Otero R (2005) Trends in the abundance of Cantabrian Capercaillie Tetrao urogallus cantabricus at leks on the southern slope of the Cantabrian Mountains, north-west Spain. Bird Conserv Int 15:397–409
Pradel R (1996) Utilization of capture-mark–recapture for the study of recruitment and population growth rate. Biometrics 52:703. https://doi.org/10.2307/2532908
Prévot-Julliard A-C, Lebreton J-D, Pradel R (1998) Re-evaluation of adult survival of Black-headed Gulls (Larus ridibundus) in presence of recapture heterogeneity. Auk 85–95
Puechmaille SJ, Petit EJ (2007) Empirical evaluation of non-invasive capture-mark–recapture estimation of population size based on a single sampling session. J Appl Ecol 44:843–852. https://doi.org/10.1111/j.1365-2664.2007.01321.x
Quevedo M, Bañuelos MJ, Obeso JR (2006) The decline of Cantabrian capercaillie: how much does habitat configuration matter? Biol Conserv 127:190–200
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86(3):248–249
Reed DH (2004) Extinction risk in fragmented habitats. Anim Conserv 7:181–191. https://doi.org/10.1017/S1367943004001313
Robertson BC, Elliott GP, Eason DK, Clout MN, Gemmell NJ (2006) Sex allocation theory aids species conservation. Biol Lett 2:229–231
Rodríguez-Muñoz R, Mirol PM, Segelbacher G, Fernández-Gil A, Tregenza T (2007) Genetic differentiation of an endangered capercaillie (Tetrao urogallus) population at the Southern edge of the species range. Conserv Genet 8:659–670
Rodríguez-Muñoz R, del Valle CR, Bañuelos MJ, Mirol P (2015) Revealing the consequences of male-biased trophy hunting on the maintenance of genetic variation. Conserv Genet 16:1375–1394. https://doi.org/10.1007/s10592-015-0747-8
Rout TM, Heinze D, McCarthy MA (2010) Optimal allocation of conservation resources to species that may be extinct. Conserv Biol 24:1111–1118. https://doi.org/10.1111/j.1523-1739.2010.01461.x
Sæther B-E, Bakke Ø (2000) Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81:642–653
Sandercock BK (2003) Estimation of survival rates for wader populations: a review of mark–recapture methods. Wader Study Group Bull 100:163–174
Schwarz CJ, Arnason AN (1996) A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 52:860. https://doi.org/10.2307/2533048
Seber GAF (1965) A note on the multiple-recapture census. Biometrika 52:249. https://doi.org/10.2307/2333827
Sedinger JS (1997) Adaptations to and consequences of a herbivorous diet in grouse and waterfowl. Condor 99:314–326
Segelbacher G, Paxton RJ, Steinbrück G, Trontelj P, Storch I (2000) Characterization of microsatellites in capercaillie Tetrao urogallus (Aves). Mol Ecol 9:1934–1936
Stephens P, Sutherland WJ (1999) Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol Evol 14:401–405
Storch I (1997) Male territoriality, female range use, and spatial organisation of capercaillie Tetrao urogallus leks. Wildl Biol 3:149–161
Storch I, Bañuelos MJ, Fernández-Gil A, Obeso JR, Quevedo M, Rodríguez-Muñoz R (2006) Subspecies Cantabrian capercaillie Tetrao urogallus cantabricus endangered according to IUCN criteria. J Ornithol 147:653–655
Suter W, Graf RF, Hess R (2002) Capercaillie (Tetrao urogallus) and avian biodiversity: testing the umbrella-species concept. Conserv Biol 16:778–788
Székely T, Weissing FJ, Komdeur J (2014) Adult sex ratio variation: implications for breeding system evolution. J Evol Biol 27:1500–1512. https://doi.org/10.1111/jeb.12415
Taberlet P, Luikart G (1999) Non-invasive genetic sampling and individual identification. Biol J Linn Soc 68:41–55
Valière N (2002) GIMLET: a computer program for analysing genetic individual identification data. Mol Ecol Notes 2(3):377–379
Van Oosterhout C, Hutchinson WF, Wills DP, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Waits LP, Luikart G, Taberlet P (2001) Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol Ecol 10:249–256
Waples RS, Do C (2010) Linkage disequilibrium estimates of contemporary Ne using highly variable genetic markers: a largely untapped resource for applied conservation and evolution. Evol Appl 3:244–262
Watson A, Moss R (2008) Grouse—the natural history of British and Irish species. HarperCollins, New York
Wegge P (1980) Distorted sex ratio among small broods in a declining Capercaillie population. Ornis Scand 11:106–109
Wegge P, Larsen B (1987) Spacing of adult and subadult male common Capercaillie during the breeding season. Auk 104:481–490
Wegge P, Larsen B, Gjerde I, Kastdalen L, Rolstad J, Storaas T (1987) Natural mortality and predation of adult capercaillie in southeast Norway. In: 4th International Grouse Symposium. World Pheasant Association, Lam, Germany
Weir LK, Grant JWA, Hutchings JA, Alonzo AESH, Shaw ERG (2011) The influence of operational sex ratio on the intensity of competition for mates. Am Nat 177:167–176. https://doi.org/10.1086/657918
Westemeier RL, Brawn JD, Simpson SA, Esker TL, Jansen RW, Walk JW, Kershner EL, Bouzat JL, Paige KN (1998) Tracking the long-term decline and recovery of an isolated population. Science 282:1695–1698
White GC (2002) Discussion comments on: the use of auxiliary variables in capture-recapture modelling. An overview. J Appl Stat 29:103–106. https://doi.org/10.1080/02664760120108476
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:S120–S139. https://doi.org/10.1080/00063659909477239
Williams KA, Frederick PC, Nichols JD (2011) Use of the superpopulation approach to estimate breeding population size: an example in asynchronously breeding birds. Ecology 92:821–828
Acknowledgements
Many collaborators helped both in the field and with the logistics of the study. We thank Eduardo González, Víctor Rodríguez and Damián Ramos for being consistently supportive of our studies. The environmental authorities of Asturias and Castilla y León granted the permits required to access capercaillie mating areas in spring. The study was funded by grant CGL2010-15990 (MICINN, Spanish Government) to MJ Bañuelos and M Quevedo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bañuelos, MJ., Blanco-Fontao, B., Fameli, A. et al. Population dynamics of an endangered forest bird using mark–recapture models based on DNA-tagging. Conserv Genet 20, 1251–1263 (2019). https://doi.org/10.1007/s10592-019-01208-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-019-01208-x