Abstract
Background
Our previous study found that B cell translocation gene 2 (BTG2) was hyper-methylated and down-regulated in side population (SP) cells of hepatocellular carcinoma (HCC) cell line. However, its clinical significances and biological impacts on HCC SP cells remained unclear.
Aims
To investigate the prognostic value of BTG2 gene in HCC and its influences on cancer stem cells (CSCs)-like traits of HCC cell line SP cells.
Methods
BTG2 expression in human HCC and adjacent non-cancerous tissues was detected by immunohistochemical staining and quantitative real-time PCR, and also obtained from GEO and TCGA data. Its prognostic values were assessed. Its biological influences on HCC cell line SP cells were evaluated using cell viability, cell cycle, plate clone-forming assay, and chemoresistance in vitro and tumorigenicity in vivo.
Results
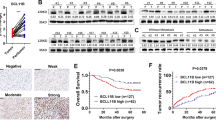
BTG2 expression was significantly suppressed in human HCC compared to adjacent non-cancerous tissues. BTG2 expression was correlated with TNM stage, tumor size and vascular invasion. Lower expression of BTG2 was associated with poorer overall survival and disease-free survival. In vitro, overexpression of BTG2 substantially suppressed cell proliferation and accumulation of HCC cell line SP cells in G0/G1 phase. Colony formation ability was markedly suppressed by BTG2 overexpression. Moreover, sensitivity of HCC cell line SP cells to 5-fluorouracil was substantially increased by overexpression of BTG2. Furthermore, tumorigenicity of HCC cell line SP cells transfected with BTG2 plasmids was significantly reduced in vivo.
Conclusions
BTG2 gene could regulate the CSC-like traits of HCC cell line SP cells, and it represented as a molecular prognostic marker for HCC.




Similar content being viewed by others
References
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111.
Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556.
Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024.
Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166.
Zhai JM, Yin XY, Hou X, et al. Analysis of the genome-wide DNA methylation profile of side population cells in hepatocellular carcinoma. Dig Dis Sci. 2013;58:1934–1947.
Boiko AD, Porteous S, Razorenova OV, Krivokrysenko VI, Williams BR, Gudkov AV. A systematic search for downstream mediators of tumor suppressor function of p53 reveals a major role of BTG2 in suppression of Ras-induced transformation. Genes Dev. 2006;20:236–252.
Mah WC, Thurnherr T, Chow PK, et al. Methylation profiles reveal distinct subgroup of hepatocellular carcinoma patients with poor prognosis. PLoS One. 2014;9:e104158.
Sung WK, Zheng H, Li S, et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genetics. 2012;44:765–769.
Burchard J, Zhang C, Liu AM, et al. microRNA-122 as a regulator of mitochondrial metabolic gene network in hepatocellular carcinoma. Mol Syst Biol. 2010;6:402.
Chang B, Li S, He Q, et al. Deregulation of Bmi-1 is associated with enhanced migration, invasion and poor prognosis in salivary adenoid cystic carcinoma. Biochim Biophys Acta. 2014;1840:3285–3291.
Duriez C, Falette N, Audoynaud C, et al. The human BTG2/TIS21/PC3 gene: genomic structure, transcriptional regulation and evaluation as a candidate tumor suppressor gene. Gene. 2002;282:207–214.
Ficazzola MA, Fraiman M, Gitlin J, et al. Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis. 2001;22:1271–1279.
Struckmann K, Schraml P, Simon R, et al. Impaired expression of the cell cycle regulator BTG2 is common in clear cell renal cell carcinoma. Cancer Res. 2004;64:1632–1638.
Kawakubo H, Brachtel E, Hayashida T, et al. Loss of B-cell translocation gene-2 in estrogen receptor-positive breast carcinoma is associated with tumor grade and overexpression of cyclin d1 protein. Cancer Res. 2006;66:7075–7082.
Wei S, Hao C, Li X, Zhao H, Chen J, Zhou Q. Effects of BTG2 on proliferation inhibition and anti-invasion in human lung cancer cells. Tumour Biol. 2012;33:1223–1230.
Hu X, Xing L, Jiao Y, et al. BTG2 overexpression increases the radiosensitivity of breast cancer cells in vitro and in vivo. Oncol Res. 2013;20:457–465.
Coppola V, Musumeci M, Patrizii M, et al. BTG2 loss and miR-21 upregulation contribute to prostate cell transformation by inducing luminal markers expression and epithelial–mesenchymal transition. Oncogene. 2013;32:1843–1853.
Park TJ, Kim JY, Oh SP, et al. TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology. 2008;47:1533–1543.
Chen Y, Chen C, Zhang Z, et al. Expression of B-cell translocation gene 2 is associated with favorable prognosis in hepatocellular carcinoma patients and sensitizes irradiation-induced hepatocellular carcinoma cell apoptosis in vitro and in nude mice. Oncol Lett. 2017;13:2366–2372.
Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284.
Sachlos E, Risueno RM, Laronde S, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297.
Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174.
Oshimori N, Oristian D, Fuchs E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160:963–976.
Van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400.
Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81472261), the Natural Science Foundation of Guangdong Province (No. 2015A030313032), the Science and Technology Development Projects of Guangdong Province, China (No. 2013B021800122), the Science and Technology Development Projects of Guangzhou, Guangdong, China (No. 201604020044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, CS., Zhai, JM., Zhu, XX. et al. BTG2 Is Down-Regulated and Inhibits Cancer Stem Cell-Like Features of Side Population Cells in Hepatocellular Carcinoma. Dig Dis Sci 62, 3501–3510 (2017). https://doi.org/10.1007/s10620-017-4829-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4829-y