Summary
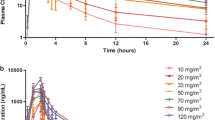
Purpose Panobinostat, a pan-deacetylase inhibitor, is a promising anti-cancer agent that increases acetylation of proteins associated with growth and survival pathways of malignant cells. The primary objective of this phase I dose-escalation study was to determine the maximum tolerated dose (MTD) of intravenous (i.v.) panobinostat administered on different dosing schedules in patients with advanced solid tumors or lymphoma. Secondary objective was to characterize safety and tolerability, pharmacokinetic profiles, and activities of the i.v. formulation. Methods i.v. panobinostat was administered at escalating doses on a daily (days 1–3 and 8–10 of a 21-day cycle; days 1–3 and 15–17 of a 28-day cycle) or weekly (days 1, 8, and 15 of a 28-day cycle; days 1 and 8 of a 21-day cycle) schedule, and safety and tolerability were monitored. Serial blood samples were collected following dosing for pharmacokinetic and pharmacodynamic analyses. Results The MTD for the daily administration schedule was 7.2 g/m2, whereas the MTD for the weekly schedule was 20.0 mg/m2. In addition to fatigue and cardiac arrhythmias, including prolonged QTcF, DLTs associated with the study drug were principally due to myelosuppressive effects. Maximum concentrations and bioavailability of i.v. panobinostat increased dose-proportionally across all doses evaluated. Conclusions Based on the results of this study and others, the i.v. formulation of panobinostat was well tolerated in many patients, but concerns remain regarding its potential suitability outside the study setting due to potential electrocardiogram abnormalities. Therefore, further development will focus on the panobinostat oral formulation.

Similar content being viewed by others
References
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325:834–840
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784
Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6:38–51
Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119:555–566
Qian DZ, Kato Y, Shabbeer S, Wei Y, Verheul HM, Salumbides B, Sanni T, Atadja P, Pili R (2006) Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res 12:634–642
Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, Ordentlich P, Wang XF, Counter CM, Yao TP (2008) The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 68:7561–7569
Kornberg RD, Lorch Y (1999) Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98:285–294
Luger K (2006) Dynamic nucleosomes. Chromosome Res 14:5–16
Johnstone RW (2002) Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 1:287–299
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1:194–202
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280:26729–26734
Yang Y, Rao R, Shen J, Tang Y, Fiskus W, Nechtman J, Atadja P, Bhalla K (2008) Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res 68:4833–4842
Gu W, Roeder RG (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595–606
Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, Pili R (2006) Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res 66:8814–8821
Verheul HM, Salumbides B, Van Erp K, Hammers H, Qian DZ, Sanni T, Atadja P, Pili R (2008) Combination strategy targeting the hypoxia inducible factor-1 alpha with mammalian target of rapamycin and histone deacetylase inhibitors. Clin Cancer Res 14:3589–3597
Maiso P, Carvajal-Vergara X, Ocio EM, Lopez-Perez R, Mateo G, Gutierrez N, Atadja P, Pandiella A, San Miguel JF (2006) The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res 66:5781–5789
Spencer A, Taylor K, Lonial S, Mateos MV, Jalaluddin M, Hazell K, Bourquelot PM, San Miguel JF (2009) Panobinostat plus lenalidomide and dexamethasone phase I trial in multiple myeloma (MM). J Clin Oncol 27 [abstract 8542]
DeAngelo DJ, Spencer A, Ottmann OG, Fischer T, Kindler T, Bhalla KN, Liu A, Parker K, Rupani H, Homji N, Ashton E, Jalaluddin M, Laird G, Scott JW, Prince HM (2009) Panobinostat has activity in treatment-refractory Hodgkin lymphoma. Haematologica 94:205 [abstract 0505]
Lin R, Hu J, Paul S, Schindler J, Woo MM, Spence S, Hirawat S, Weber HA (2009) Characteristics of thrombocytopenia in patients treated with oral panobinostat (LBH589). Blood 114 [abstract 2740]
Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, Masson E, Rae P, Laird G, Sharma S, Kantarjian H, Dugan M, Albitar M, Bhalla K (2006) A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 12:4628–4635
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89:1138–1147
Neuenschwander B, Branson M, Gsponer T (2008) Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med 27:2420–2439
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Bunn PA Jr, Lamberg SI (1979) Report of the committee on staging and classification of cutaneous T-cell lymphomas. Cancer Treat Rep 63:725–728
Foss F (2003) Overview of cutaneous T-cell lymphoma: prognostic factors and novel therapeutic approaches. Leuk Lymphoma 44(Suppl 3):S55–S61
Whittaker SJ, Marsden JR, Spittle M, Russell Jones R, British Association of Dermatologists, U.K., Cutaneous Lymphoma Group (2003) Joint British Association of Dermatologists and U.K. cutaneous lymphoma group guidelines for the management of primary cutaneous T-cell lymphomas. Br J Dermatol 149:1095–1107
Hamberg P, Woo MM, Chen LC, Verweij J, Porro MG, Zhao L, Li W, van der Biessen D, Sharma S, Hengelage T, de Jonge M (2011) Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor. Cancer Chemother Pharmacol
Ellis L, Pan Y, Smyth GK, George DJ, McCormack C, Williams-Truax R, Mita M, Beck J, Burris H, Ryan G, Atadja P, Butterfoss D, Dugan M, Culver K, Johnstone RW, Prince HM (2008) Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res 14:4500–4510
Babb J, Rogatko A, Zacks S (1998) Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med 17:1103–1120
Munster PN, Rubin EH, Van Belle S, Friedman E, Patterson JK, Van Dyck K, Li X, Comisar W, Chodakewitz JA, Wagner JA, Iwamoto M (2009) A single supratherapeutic dose of vorinostat does not prolong the QTc interval in patients with advanced cancer. Clin Cancer Res 15:7077–7084
Piekarz RL, Frye AR, Wright JJ, Steinberg SM, Liewehr DJ, Rosing DR, Sachdev V, Fojo T, Bates SE (2006) Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res 12:3762–3773
Wagner JM, Hackanson B, Lubbert M, Jung M (2010) Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics 1:117–136
Chiang CE (2004) Congenital and acquired long QT syndrome. Current concepts and management. Cardiol Rev 12:222–234
Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, Duvic M (2007) Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 25:3109–3115
Rowinsky EK, de Bono J, Deangelo DJ, van Oosterom A, Morganroth J, Laird GH, Dugan M, Scott JW, Ottmann OG (2005) Cardiac monitoring in phase I trials of a novel histone deacetylase (HDAC) inhibitor LAQ824 in patients with advanced solid tumors and hematologic malignancies. J Clin Oncol 23 [abstract 3131]
Zhang L, Lebwohl D, Masson E, Laird G, Cooper MR, Prince HM (2008) Clinically relevant QTc prolongation is not associated with current dose schedules of LBH589 (panobinostat). J Clin Oncol 26:332–333, discussion 333–4
Weber HA, Tai F, Paul S, Schindler J, Woo MM, Spence S, Marlowe J, Lin R (2009) QT interval measurements in patients with hematologic malignancies and solid tumors treated with oral panobinostat (LBH589). Blood 114 [abstract 3781]
Bates SE, Zhan Z, Steadman K, Obrzut T, Luchenko V, Frye R, Robey RW, Turner M, Gardner ER, Figg WD, Steinberg SM, Ling A, Fojo T, To KW, Piekarz RL (2010) Laboratory correlates for a phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br J Haematol 148:256–267
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Kerry K. Brinkman, Ph.D., for medical editorial assistance with this manuscript.
Conflict of interest
J.B., M.M.: Nothing to disclose. H.M.P.: Novartis consultancy, research funding, and honoraria. S.S.: Novartis consultancy and research funding. B.G., S.P., M.S., M.W.: Novartis employment. M.W.: Novartis equity ownership.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Sharma, S., Beck, J., Mita, M. et al. A phase I dose-escalation study of intravenous panobinostat in patients with lymphoma and solid tumors. Invest New Drugs 31, 974–985 (2013). https://doi.org/10.1007/s10637-013-9930-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9930-2