Abstract
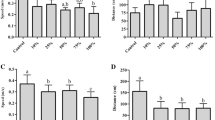
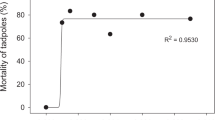
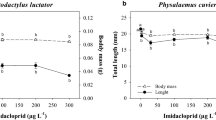
Cadmium (Cd) exposure is harmful to amphibians in natural environments and the Cd concentration is a key parameter in water monitoring. Cd pollution has been a severe issue in the Yangtze River and its southern reaches in recent years. Acute toxicity assays were employed to determine the tolerance limits of Cd for Microhyla fissipes tadpoles and five different concentrations of Cd (0, 50, 100, 200 and 300 μg/L) were involved to detect its chronic effects on metamorphosis, growth, locomotion, genotoxicity and enzymatic activities of M. fissipes tadpoles. The results showed that the 24-h and 48-h LC50 values of Cd on M. fissipes tadpoles were 2591.3 μg/L and 1567.9 μg/L, respectively, and the presumable non-lethal concentration obtained was 172.2 μg/L. During the 70-day chronic toxicity assays, Cd showed negative impacts on survival, growth, metamorphosis and the frequency of erythrocytes nuclear abnormality of M. fissipes tadpoles. However, the Cd exposure caused the increased body size and condition of tadpoles at complete metamorphosis (GS46). The tadpoles exposed to 200 μg/L of Cd exhibited degraded locomotor performance at GS46. Weight increments of tadpoles were inhibited at Day 14 and massive deaths were observed over the next 14 days. The enzymatic activities of tadpoles experienced a shock response stage (GS30-GS35) and a complete recovery stage (GS36-GS41) in all treatments. However, the enzymatic activities (except alkaline phosphatase) of tadpoles at GS46 increased after Cd exposure, especially at high concentrations. In summary, Cd is a threat to M. fissipes tadpoles as that causes reduced fitness.




Similar content being viewed by others
References
Barfield ML, Farris JL, Black MC (2001) Biomarker and bioaccumulation responses of Asian clams exposed to aqueous cadmium. J Toxicol Env Heal A 63:495–510
Barni S, Boncompagni E, Grosso A, Bertone V, Freitas I, Fasola M, Fenoglio C (2007) Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquat Toxicol 81:45–54
Bliss CI (1934) The method of probits. Science 79:38–39
Brito R, Rosas C, Chimal M, Gaxiola G (2001) Effect of different diets on growth and digestive enzyme activity in Litopenaeus vannamei (Boone 1931) early post‐larvae. Aquac Res 32:257–266
Brodeur JC, Sassone A, Hermida GN, Codugnello N (2013) Environmentally-relevant concentrations of atrazine induce non-monotonic acceleration of developmental rate and increased size at metamorphosis in Rhinella arenarum tadpoles. Ecotox Environ Safe 92:10–17
Cabrera-Guzmán E, Crossland MR, Brown GP, Shine R (2013) Larger body size at metamorphosis enhances survival, growth and performance of young cane toads (Rhinella marina). PLoS ONE 8:e70121
Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A (2000) The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: a practical approach. Sci Total Environ 247:295–311
Carrasco KR, Tilbury KL, Myers MS (1990) Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Can J Fish Aquat Sci 47:2123–2136
Dal-Medico SE, Rissoli RZ, Gamero FU, Victório JA, Salla RF, Abdalla FC, Silva-Zacarin ECM, Carvalho CS, Costa MJ (2014) Negative impact of a cadmium concentration considered environmentally safe in Brazil on the cardiac performance of bullfrog tadpoles. Ecotox Environ Safe 104:168–174
Ding GH, Lin ZH, Fan XL, Ji X (2015) The combined effects of food supply and larval density on survival growth and metamorphosis of Chinese tiger frog (Hoplobatrachus rugulosa) tadpoles. Aquaculture 435:398–402
Dodd MHI, Dodd JM (1976) The biology of metamorphosis. In: Lofts B (ed) Physiology of the Amphibia. Academic Press, New York, pp. 467–599
Duellman WE, Trueb L (1986) Biology of amphibians. McGraw-Hill, New York, pp. 670
Fei L, Ye CY, Jiang JP (2012) Colored atlas of Chinese amphibians and their distributions. SichuanScience and Technology Press, Sichuan, pp. 572–573
Fenech M (2000) The in vitro micronucleus technique. Mutat Res-Fund Mol M 455:81–95
Forget J, Pavillon JF, Beliaeff B, Bocquené G (1999) Joint action of pollutant combinations (pesticides and metals) on survival (LC50 values) and acetylcholinesterase activity of Tigriopus brevicornis (Copepoda Harpacticoida). Environ Toxicol Chem 18:912–918
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Greville RW, Morgan AJ (1991) A comparison of (Pb, Cd and Zn) accumulation in terrestrial slugs maintained in microcosms: evidence for metal tolerance. Environ Pollut 74:115
Gross JA, Chen TH, Karasov WH (2007) Lethal and sublethal effects of chronic cadmium exposure on northern leopard frog (Rana pipiens) tadpoles. Environ Toxicol Chem 26:1192–1197
Guilherme S, Válega M, Pereira M, Santos M, Pacheco M (2008) Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotox Environ Safe 70:411–421
Hart W, Weston RF, Demann J (1948) An apparatus for oxygenating test solutions in which fish are used as test animals for evaluating toxicity. T Am Fish Soc 75:228–236
Haywood LK, Alexander GJ, Byrne MJ, Cukrowska E (2004) Xenopus laevis embryos and tadpoles as models for testing for pollution by zinc, copper, lead and cadmium. Afr Zool 39:163–174
Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, Tucker JD, Vanparys PH, MacGregor JT (1991) Micronuclei as an index of cytogenetic damage: past, present, and future. Environ Mol Mutagen 18:277–291
Herkovits J, Cardellini P, Pavanati C, Perez‐Coll CS (1997) Susceptibility of early life stages of Xenopus laevis to cadmium. Environ Toxicol Chem 16:312–316
Hopkins WA (2007) Amphibians as models for studying environmental change. Ilar J 48:270
Jakimska A, Konieczka P, Skóra K, Namieśnik J (2011) Bioaccumulation of metals in tissues of marine animals, part I: the role and impact of heavy metals on organisms. Pol J Environ Stud 20:1117–1125
James SM, Little EE (2003) The effects of chronic cadmium exposure on American toad (Bufo americanus) tadpoles. Environ Toxicol Chem 22:377–380
James SM, Little EE, Semlitsch RD (2005) Metamorphosis of two amphibian species after chronic cadmium exposure in outdoor aquatic mesocosms. Environ Toxicol Chem 24:1994–2001
James SM, Semlitsch RD (2011) Terrestrial performance of juvenile frogs in two habitat types after chronic larval exposure to a contaminant. J Herpetol 45:186–194
Johansson M, Piha H, Kylin H, Merilä J (2006) Toxicity of six pesticides to common frog (Rana temporaria) tadpoles. Environ Toxicol Chem 25:3164–3170
Kerby JL, Richards‐Hrdlicka KL, Storfer A, Skelly DK (2010) An examination of amphibian sensitivity to environmental contaminants: are amphibians poor canaries? Ecol Lett 13:60–67
Khangarot BS, Sehgal A, Bhasin MK (1985) “Man and biosphere”-studies on the Sikkim Himalayas. part 5: acute toxicity of selected heavy metals on the tadpoles of Rana hexadactyla. Clean-Soil Air Water 13:259–263
Lefcort H, Meguire R, Wilson L, Ettinger W (1998) Heavy metals alter the survival, growth, metamorphosis, and antipredatory behavior of Columbia spotted frog (Rana luteiventris) tadpoles. Arch Environ Con Tox 35:447–456
Liang LN, Hu JT, Chen DY, Zhou QF, He B, Jiang GB (2004) Primary investigation of heavy metal contamination status in mollusks collected from Chinese coastal sites. Bull Environ Contam Tox 72:937–944
Liao JB, Chen J, Ru X, Chen JD, Wu HZ, Wei CH (2017) Heavy metals in river surface sediments affected with multiple pollution sources, South China: distribution enrichment and source apportionment. J Geochem Explor 176:9–19
Liu ZP, Zhang QF, Han TQ, Ding YF, Sun JW, Wang FJ, Zhu C (2015) Heavy metal pollution in a soil-rice system in the Yangtze river region of China. Int J Env Res Pub He 13:63
Loumbourdis NS, Kyriakopoulou-Sklavounou P, Zachariadis G (1999) Effects of cadmium exposure on bioaccumulation and larval growth in the frog Rana ridibunda. Environ Pollut 104:429–433
Luo JM, Yin XR, Ya YJ, Wang YJ, Zang SY, Zhou X (2013) Pb and Cd bioaccumulations in the habitat and preys of red-crowned cranes (Grus japonensis) in Zhalong wetland, northeastern China. Biol Trace Elem Res 156:134–143
Luo ZH, Tian DL, Ning C, Yan W, Xiang WH, Peng CH (2015) Roles of Koelreuteria bipinnata as a suitable accumulator tree species in remediating Mn, Zn, Pb, and Cd pollution on Mn mining wastelands in southern China. Environ Earth Sci 74:4549–4559
Marques SM, Antunes SC, Pissarra H, Pereira ML, Gonçalves F, Pereira R (2009) Histopathological changes and erythrocytic nuclear abnormalities in Iberian green frogs (Rana perezi Seoane) from a uranium mine pond. Aquat Toxicol 91:187–195
Marques SM, Antunes SC, Nunes B, Goncalves F, Pereira R (2011) Antioxidant response and metal accumulation in tissues of Iberian green frogs (Pelophylax perezi) inhabiting a deactivated uranium mine. Ecotoxicology 20:1315–1327
Martelli A, Rousselet E, Dycke C, Bouron A, Moulis JM (2006) Cadmium toxicity in animal cells by interference with essential metals. Biochimie 88:1807–1814
McDonald DG, Wood CM (1993) Fish ecophysiology. In: Rankin JC, Jensen FB (eds) Branchial mechanisms of acclimation to metals in freshwater fish. Chapman and Hall, London, pp. 297–321
Miao X, Tang YH, Wong CWY, Zang HY (2015) The latent causal chain of industrial water pollution in China. Environ Pollut 196:473–477
Mouchet F, Baudrimont M, Gonzalez P, Cuenot Y, Bourdineaud JP, Boudoub A, Gauthier L (2006) Genotoxic and stress inductive potential of cadmium in Xenopus laevis larvae. Aquat Toxicol 78:157–166
Mouchet F, Gauthier L, Baudrimont M, Gonzalez P, Mailhes C, Ferrier V, Devaux A (2007) Comparative evaluation of the toxicity and genotoxicity of cadmium in amphibian larvae (Xenopus laevis and Pleurodeles waltl) using the comet assay and the micronucleus test. Environ Toxicol 22:422–435
Chalkiadaki O, Dassenakis M, Lydakis-Simantiris N (2014) Bioconcentration of Cd and Ni in various tissues of two marine bivalves living in different habitats and exposed to heavily polluted seawater. Chem Ecol 30:726–742
Ossana NA, Castañé PM, Poletta GL, Mudry MD, Salibián A (2010) Toxicity of waterborne copper in premetamorphic tadpoles of Lithobates catesbeianus (Shaw 1802). Bull Environ Contam Tox 84:712–715
Özkan F, Gündüz SG, Berköz M, Hunt AÖ (2011) Induction of micronuclei and other nuclear abnormalities in peripheral erythrocytes of Nile tilapia, Oreochromis niloticus, following exposure to sublethal cadmium doses. Turk J Zool 35:585–592
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421:3–16
Patar A, Giri A, Boro F, Bhuyan K, Singha U, Giri S (2016) Cadmium pollution and amphibians–Studies in tadpoles of Rana limnocharis. Chemosphere 144:1043–1049
Peles JD, Pistole DH, Moffe M (2012a) Influence of cadmium concentration and length of exposure on metabolic rate and gill Na+/K+ ATPase activity of golden shiners (Notemigonus crysoleucas). Comp Biochem Phys C 156:24–28
Peles JD, Pistole DH, Moffe MC (2012b) Time-specific and population-level differences in physiological responses of fathead minnows (Pimephales promelas) and golden shiners (Notemigonus crysoleucas) exposed to copper. Aquat Toxicol 109:222–227
Ranatunge RAAR, Wijesinghe MR, Ratnasooriya WD, Dharmarathne HASG, Wijesekera RD (2012) Cadmium-induced toxicity on larvae of the common Asian toad Duttaphrynus melanostictus (Schneider 1799): evidence from empirical trials. Bull Environ Contam Toxicol 89:143–146
Rocha TL, Gomes T, Cardoso C, Letendre J, Pinheiro JP, Sousa VS, Teixeira MR, Bebianno MJ (2014) Immunocytotoxicity, cytogenotoxicity and genotoxicity of cadmium-based quantum dots in the marine mussel Mytilus galloprovincialis. Mar Environ Res 101:29–37
Sharma B, Patiño R (2008) Exposure of Xenopus laevis tadpoles to cadmium reveals concentration-dependent bimodal effects on growth and monotonic effects on development and thyroid gland activity. Toxicol Sci 105:51–58
Sharma B, Patiño R (2009) Effects of cadmium on growth, metamorphosis and gonadal sex differentiation in tadpoles of the African clawed frog, Xenopus laevis. Chemosphere 76:1048–1055
Smith PN, Cobb GP, Godard-Codding C, Hoff D, Mcmurry ST, Rainwater TR, Reynolds KD (2007) Contaminant exposure in terrestrial vertebrates. Environ Pollut 150:41–64
Strong RJ, Halsall CJ, Ferenčík M, Jones KC, Shore RF, Martin FL (2016) Biospectroscopy reveals the effect of varying water quality on tadpole tissues of the common frog (Rana temporaria). Environ Pollut 213:322–337
Sun NL, Wang HY, Ju ZQ, Zhao HF (2018) Effects of chronic cadmium exposure on metamorphosis, skeletal development, and thyroid endocrine disruption in Chinese toad Bufo gargarizans tadpoles. Environ Toxicol Chem 37:213–223
Tata JR (2006) Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol 246:10–20
Wang LH, Jiao YM, Ming QZ, He LL, Zhou HB (2009) Evaluation of heavy metal pollution in Bijiang Basin in Yunnan Province. Res. Environ Sci 22:595–606
Wake DB, Vredenburg VT (2008) Colloquium paper: are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA 105:11466–11473
Wallace RA (1961) Enzymatic patterns in the developing frog embryo. Dev Bio 3:486–515
Wang C, Liang G, Chai L, Wang H (2016) Effects of copper on growth, metamorphosis and endocrine disruption of Bufo gargarizans larvae. Aquat Toxicol 170:24–30
Watkins TB (1996) Predator-mediated selection on burst swimming performance in tadpoles of the Pacific tree frog, Pseudacris regilla. Physiol Zool 69:154–167
Wei L, Ding GH, Guo SN, Tong ML, Chen WJ, Flanders J, Shao WW, Lin ZH (2015) Toxic effects of three heavy metallic ions on Rana zhenhaiensis tadpoles. Asian Herpetol Res 6:132–142
Wu C, Zhang YH, Chai LH, Wang HY(2017) Histological changes, lipid metabolism and oxidative stress in the liver of Bufo gargarizans exposed to cadmium concentrations Chemosphere 179:337–346
Wu QH, Leung JYS, Geng XH, Chen SJ, Huang XX, Li HY, Huang ZY, Zhu LB, Chen JH, Lu YY (2015) Heavy metal contamination of soil and water in the vicinity of an abandoned e-waste recycling site: implications for dissemination of heavy metals. Sci Total Environ 506:217–225
Xu XH, Zhao YC, Zhao XY, Wang YD, Deng WJ (2014) Sources of heavy metal pollution in agricultural soils of a rapidly industrializing area in the Yangtze Delta of China. Ecotox Environ Safe 108:161–167
Zhao YY, Fang XL, Mu YH, Cheng YB, Ma QB, Nian H, Yang CY (2014) Metal pollution (Cd, Pb, Zn, and As) in agricultural soils and soybean, glycine max, in southern China. Bull Environ Contam Tox 92:427–432
Acknowledgements
This work was supported by grants from Zhejiang Provincial Natural Science Foundation of China (LQ16C040001, Y20C030006), National Science Foundation of China (31500308) and Zhejiang Science and Technology Innovation Program for College Students (2019R434006). We thank Sai-Nan Guo, Wen-Jun Chen, Mei-Ling Tong, Guo-Qing Cai for their help during the research.
Author contributions
YT, YCH, and GHD performed statistical analysis and wrote the paper. YCH and GHD conceived the idea and supervised the study, ZQC and JYC captured and raised the animals, YT and YCH collected the data. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ying-Chao Hu, Yun Tang
Rights and permissions
About this article
Cite this article
Hu, YC., Tang, Y., Chen, ZQ. et al. Evaluation of the sensitivity of Microhyla fissipes tadpoles to aqueous cadmium. Ecotoxicology 28, 1150–1159 (2019). https://doi.org/10.1007/s10646-019-02117-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02117-y