Abstract
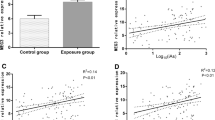
Arsenic exposure could induce apoptosis and cause related cancer. It was reported that p38 signaling pathway played a key transcriptional regulatory factor in arsenic-induced apoptosis. However, there were certain disputable questions about this point of opinion. Therefore, the relationship between p38 signaling pathway and arsenic-induced apoptosis was systematically reviewed and analyzed by meta-analysis. Twelve essays were analyzed with StataSE15.0 and Review Manager 5.3. The regulatory variables, such as normal cells and cancer cells, arsenic exposure time and exposure dose were analyzed by the subgroup analysis. The comprehensive effects were compared and analyzed by SMD method. Publication bias, the monolithic impact and heterogeneity were inspected. Subgroup analysis showed, when arsenic exposure was ≥ 5 μmol/l, the expression of Bcl-2 and Bax was down-regulated and the expression of p38 and Caspase-3 was up-regulated. When arsenic exposure was < 5 μmol/l, the expression of Bcl-2, Bax, p38 and Caspase-3 was up-regulated. Arsenic exposure time (≥ 48 h) or arsenic exposure dose (≥ 5 μmol/l or < 5 μmol/l) can promote the expression of p38. Arsenic exposure time was ≥ 48 h or exposure dose was < 5 μmol/l in cancer cells, arsenic exposure dose was ≥ 5 μmol/l or exposure time was < 48 h in normal cells, and they are statistically significant in the expression of p38. This study evaluates the role of p38 signaling pathway in arsenic-induced apoptosis, which is helpful to provide theoretical basis for the differentiation of arsenic-induced injury and the therapeutic mechanism of arsenic-induced apoptosis.











Similar content being viewed by others
References
Ahmed, M. K., Shaheen, N., et al. (2015). Dietary intake of trace elements from highly consumed cultured fifish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere, 1, 1. https://doi.org/10.1016/j.chemosphere.2015.02.016.
Akanda, R., In-Shik, K., Dongchoon, A., et al. (2017). In vivo and in vitro hepatoprotective effects of Geranium koreanum, methanolic extract via downregulation of MAPK/caspase-3 pathway [J]. Evidence-Based Complementary and Alternative Medicine, 2017, 1–12. https://doi.org/10.1155/2017/8137627.
Alice, M., Stevens, J. J., Ndebele, K., et al. (2010). Arsenic trioxide modulates DNA synthesis and apoptosis in lung carcinoma cells [J]. International Journal of Environmental Research and Public Health, 7(5), 1996–2007. https://doi.org/10.3390/ijerph7051996.
Benbrahim-Tallaa, L., & Waalkes, M. P. (2008). Inorganic arsenic and human prostate cancer. Environmental Health Perspectives, 116(2), 158–164. https://doi.org/10.1289/ehp.10423.
Chang, Hong, & Guangfu, Zhang. (2015). Study on antitumor effect of arsenic trioxide combined with tanshinone capsule on hepatoma cell line bel-7404. Chinese Journal of Traditional Chinese Medicine, 30(11), 3881–3885.
Cheremisinoff, N. P. (2016). Agency for toxic substances and disease registry (ATSDR). Pollution control handbook for oil and gas engineering. Hoboken: Wiley. https://doi.org/10.1002/9781119117896.ch1.
Chowdhuri R., Chowdhury S., Roychoudhury P., et al. (2009). Arsenic induced apoptosis in malignant melanoma cells is enhanced by menadione through ROS generation, p38 signaling and p53 activation [J]. Apoptosis, 14(1), 108–123. https://doi.org/10.1007/s10495-008-0284-8.
Das, J., Ghosh, J., Manna, P., & Sil, P. C. (2010). Protective role of taurine against arsenic-induced mitochondria dependent hepatic apoptosis via the inhibition of PKCdelta-JNK pathway. PLoS ONE, 5(9), 1. https://doi.org/10.1371/journal.pone.0012602.
Davison, K., Mann, K. K., Waxman, S., & Miller, W. J. (2004). JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood, 103, 3496–3502. https://doi.org/10.1182/blood-2003-05-1412.
Dent, P., & Grant, S. (2001). Pharmacologic interruption of the mitogen-activated extracellular-regulated kinase/mitogen-activated protein kinase signal transduction pathway: Potential role in promoting cytotoxic drug action. Clinical Cancer Research, 7(4), 775–783. https://doi.org/10.1093/carcin/22.4.681.
Dingar, D., Merlen, C., Grandy, S., Gillis, M. A., Villeneuve, L. R., Mamarbachi, A. M., et al. (2010). Effect of pressure overload-induced hypertrophy on the expression and localization of p38 MAP kinase isoforms in the mouse heart. Cellular Signalling, 1, 221634–221644. https://doi.org/10.1016/j.cellsig.2010.06.002.
Dong, Xiaolin, Jie, Yang, & Shaojun, Zhang. (2017). Expression and significance of MAPK/AKT in apoptosis of Jurkat lymphoma induced by arsenic trioxide. Anatomical Study, 39(02), 115–118.
Eguchi, R., Fujimori, Y., Takeda, H., et al. (2011). Arsenic trioxide induces apoptosis through JNK and ERK in human mesothelioma cells. Journal of Cellular Physiology, 226(3), 762–768. https://doi.org/10.1002/jcp.22397.
Fan, M., & Chambers, T. C. (2001). Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resistance Updates, 4, 253–267. https://doi.org/10.1054/drup.2001.0214.
Giafifis, N., Katsoulidis, E., Sassano, A., Tallman, M. S., Higgins, L. S., Nebreda, A. R., et al. (2006). Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Research, 66, 6763–6771. https://doi.org/10.1158/0008-5472.can-05-3699.
IARC (International Agency for Research on Cancer). (2004). Some drinking water disinfectants and contaminants, including arsenic. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 84(2004), 269–477.
Jyotirmoy, Ghosh, Joydeep, Das, Prasenjit, Manna, et al. (2009). Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: Role of NF-kappa B, p38 and JNK MAPK pathway. Toxicology and Applied Pharmacology, 240, 73–87. https://doi.org/10.1016/j.taap.2009.07.008.
Kim, H. G., Shi, C., Bode, A. M., et al. (2016). p38α MAPK is required for arsenic-induced cell transformation. Molecular Carcinogenesis, 55(5), 910–917. https://doi.org/10.1002/mc.22331.
Liu, Y., Hock, J. M., Sullivan, C., et al. (2010). Activation of the p38. MAPK/Akt/ERK1/2 signal pathways is required for the protein stabilization of CDC6 and cyclin D1 in low-dose arsenite-induced cell proliferation. Journal of Cellular Biochemistry, 111(6), 1546–1555. https://doi.org/10.1002/jcb.22886.
Liu, G., Zhang, V., Bode, A. M., Ma, W. Y., & Dong, Z. (2001). Phosphorylation of 4E-BP1 is mediated by the p38/MSK1 pathway in response to UVB irradiation. Journal of Biological Chemistry, 277, 8810–8816. https://doi.org/10.1074/jbc.M110477200.
Luo, Yanhong, Yaodong, Wei, Taizhong, Wang, Tiansheng, Lu, Ruibo, Wu, Dongzhu, Chen, et al. (2011). Effects of pine pollen on apoptosis-related factors and proteins in cerebral cortex of mice with arsenism. Journal of Environment and Health, 28(07), 586–588.
Miao, X., Tang, Z., et al. (2013). Metallothionein prevention of arsenic trioxide induced cardiac cell death is associated with its inhibition of mitogen activated protein kinases activation in vitro and in vivo. Toxicology Letters, 220, 277–285. https://doi.org/10.1016/j.toxlet.2013.04.02.
Namgung, U., & Xia, Z. (2001). Arsenic Induces Apoptosis in Rat Cerebellar Neurons via Activation of JNK3 and p38 MAP Kinases. Toxicology and Applied Pharmacology, 174(2), 130–138. https://doi.org/10.1006/taap.2001.9200.
Organization, W. H. (2004). Some drinking-water disinfectants and contaminants, including arsenic. Monographs on chloramine, chloral and chloral hydrate, dichloroacetic acid, trichloroacetic acid and 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 84, 1.
Qu, W. (2002). Acquisition of apoptotic resistance in arsenic-induced malignant transformation: Role of the JNK signal transduction pathway. Carcinogenesis, 23(1), 151–159. https://doi.org/10.1093/carcin/23.1.151.
Ray, A., Chatterjee, S., Mukherjee, S., et al. (2013). Interplay of loss of ERK dependence and amplification of apoptotic signals in arsenic treated rat hepatocytes. National Academy Science Letters, 36(6), 599–602. https://doi.org/10.1007/s40009-013-0175-6.
Rodríguez-Sosa, Miriam, García-Montalvo, Eliud A., Razo, Luz María Del, et al. (2013). Effect of selenomethionine supplementation in food on the excretion and toxicity of arsenic exposure in female mice. Biological Trace Element Research, 156(1), 279–287. https://doi.org/10.1007/s12011-013-9855-9.
Rojewski, M. T., Korper, S., Thiel, E., & Schrezenmeir, H. (2004). Arsenic trioxide induced apoptosis is independent of CD95 in lymphocytic cell. Oncology Reports, 11, 509–513. https://doi.org/10.3892/or.11.2.509.
Ruemmele, F. M., Dionne, S., Qureshi, I., et al. (1999). Butyrate mediates Caco-2 cell apoptosis via up-regulation of pro-apoptotic BAK and inducing caspase-3 mediated cleavage of poly-(ADP-ribose) polymerase (PARP). Cell Death and Differentiation, 1999(6), 729–735. https://doi.org/10.1038/sj.cdd.4400545.
Sarkar, D., Su, Z. Z., Lebedeva, I. V., et al. (2002). mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proceedings of the National Academy of Sciences, 99(15), 10054–10059. https://doi.org/10.1073/pnas.152327199.
Soma, Datta, Debabrata, Ghosh, Rani, Saha Dhira, et al. (2009). Chronic exposure to low concentration of arsenic is immunotoxic to fish: Role of head kidney macrophages as biomarkers of arsenic toxicity to Clarias batrachus. Aquatic Toxicology. https://doi.org/10.1016/j.aquatox.2009.01.002.
Su, Xiaoming, Jiang, Tao, Zheng, Lei, Peng, Jinqiang, Sun, Dongchen, Li, Quanlin, et al. (2013). Study on P38 signal pathway of apoptosis in prostate cancer PC-3 cells induced by arsenic trioxide. Chinese Journal of Andrology, 19(07), 583–587.
Tallman, M. S. (2001). Arsenic trioxide: Its role in acute promyelocytic leukemia and potential in other hematologic malignancies. Blood Reviews, 15, 133–142. https://doi.org/10.1054/blre.2001.0160.
Wenbin, Xu, Wei, Wei, Qing, Yu., et al. (2014). Arsenic trioxide and bortezomib interact synergistically to induce apoptosis in chronic myelogenous leukemia cells resistant to imatinib mesylate through Bcr/Abldependent mechanisms. Molecular Medicine Reports, 10, 1519–1524. https://doi.org/10.3892/mmr.2014.2333.
Xiaowei, Gong, & Yong, Jiang. (2003). Structural basis of biological function of mitogen-activated protein kinase (MAPK). Chinese Journal of Biochemistry and Molecular Biology, 1, 1. https://doi.org/10.3969/j.issn.1007-7626.2003.01.002.
Xinyang, Li, Donglei, Sun, Tianhe, Zhao, et al. (2020). Long non-coding RNA ROR confers arsenic trioxide resistance to HepG2 cells by inhibiting p53expression. European Journal of Pharmacology, 872, 172982. https://doi.org/10.2147/OTT.S225301.
Xuyi, Zhou, & Jiqian, Fang. (2002). common prejudices of meta analysis. Evidence-Based Medicine, 2(4), 216–220.
Yan, Xia, Xianhao, Liu, Beibei, Liu, et al. (2018). Enhanced antitumor activity of combined megestrol acetate and arsenic trioxide treatment in liver cancer cells. Experimental and Therapeutic Medicine, 15(4), 4047–4055. https://doi.org/10.3892/etm.2018.5905.
Yang, Yiju, & Zhenqiu, Sun. (2012). Puerarin and arsenic trioxide promote apoptosis of human glioma cells through Akt/p38 pathway. Clinical Oncology in China, 39(24), 2059–2062. https://doi.org/10.3969/j.issn.1000-8179.2012.24.018.
Yuan, Fangfang, Xuhua, Zhang, Ruihua, Mi, Ruihua, Fan, Qingsong, Yin, & Xudong, Wei. (2014). Experimental study on the effect of arsenic trioxide combined with TPA on K562 cells. Chinese Journal of Experimental Hematology, 22(04), 943–949. https://doi.org/10.7534/j.issn.1009-2137.2014.04.012.
Zhang, Jing-Yi, Sun, Gui-Bo, Luo, Yun et al. (2017). Salvianolic acid a protects H9c2 cells from arsenic trioxide-induced injury via inhibition of the MAPK signaling pathway [J]. Journal of Cellular Physiology Biochemistry, 41, 1957–1969. https://doi.org/10.1159/000472409.
Acknowledgement
The authors would like to thank all the people who helped us with this work.
Funding
Funding was provided by National Natural Science Foundation of China (NSFC), Numbers: 81860560, 81660835 and 81160336, and the open project of State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University (FAMP201802K).
Author information
Authors and Affiliations
Contributions
Authors’ contributions
LWU did the most of the paper on meta-analysis; Dr. PL and XL made contributions on data collection and reviewed the methods and results and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Availability of data and material
Our data are from the website, so in our study we used collected data for the research.
Ethics approval and consent to participate
This article does not contain any studies with human or animal subjects performed by any of the authors. All participants gave informed consent before taking part in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, L., Li, X., Wei, S. et al. Relationship between p38 signaling pathway and arsenic-induced apoptosis: a meta-analysis. Environ Geochem Health 43, 1213–1224 (2021). https://doi.org/10.1007/s10653-020-00646-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00646-8