Abstract
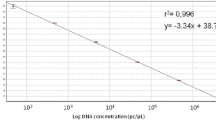
Pratylenchus neglectus and P. thornei are economically important migratory plant endoparasitic nematodes and are widely found in a wide variety of crops. Based on 28 s rDNA D2D3 expansion region, a duplex real-time quantitative (qPCR) assay was developed to simultaneously detect and quantify these two nematodes. The qPCR system included two pairs of primers and two Taqman probes, that is the P. neglectus-specific primer pair 28sPnF5/28sPnR7 and probe pntaq3 and the P. thornei-specific primer pair 28sPtF1/28sPtR1 and probe pttaq3. The optimal conditions of the qPCR assay were 20 μL reaction volume comprising 10 μL THUNDERBIRD Probe qPCR Mix, 0.2 μM of primer 28sPnF5 and 28sPnR7, 0.25 μM of primer 28sPtF1and 28sPtR1, 0.1 μM of probe pntaq3 and 0.08 μM of probe pttaq3, 1 μL template DNA. And the amplification program were 95 °C for 2 min, 40 cycles of 95 °C for 10s, 65 °C for 25 s. The specificity of the qPCR assay was confirmed by the lack of fluorescence signal from non-target nematode populations including seven other Pratylenchus species and five other nematode species. The assay was very sensitive as it can detect a single target nematode and a single target nematode mixed with up to 100 individuals of non-target nematodes. A dilution series from P. neglectus and P. thornei DNAs resulted in two standard curves respectively showing a highly significant linearity between the Cycle threshold (Ct) values and the dilution rates. This study is the first to provide a molecular tool for simultaneous detection and quantification of P. neglectus and P. thornei.




Similar content being viewed by others
References
Al-Banna, L., Ploeg, A. T., Williamson, V. M., & Kaloshian, I. (2004). Discrimination of six Pratylenchus species using PCR and species-specific primers. Journal of Nematology, 36(2), 142–146.
Berry, S. D., Fargette, M., Spaull, V. W., Morand, S., & Cadet, P. (2008). Detection and quantification of root-knot nematode (Meloidogyne javanica), lesion nematode (Pratylenchus zeae) and dagger nematode (Xiphinema elongatum) parasites of sugarcane using real-time PCR. Molecular and Cellular Probes, 22(3), 168–176.
Carrasco-Ballesteros, S., Castillo, P., Adams, B. J., & Perez-Artes, E. (2007). Identification of Pratylenchus thornei, the cereal and legume root-lesion nematode, based on SCAR-PCR and satellite DNA. European Journal of Plant Pathology, 118(2), 115–125.
Castillo, P., & Vovlas, N. (2007). Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, biology, pathogenicity and management. In D. J. Hunt & R. N. Perry (Eds.), Nematology monographs and perspectives 6 (p. 529). Leiden: Brill.
Castillo, P., Trapero-Casas, J. L., & Jiménez-Díaz, R. M. (1995). Effect of time, temperature, and inoculum density on reproduction of Pratylenchus thornei in carrot disk cultures. Journal of Nematology, 27(1), 120–124.
Dababat, A. A., Ferney, G. H., Erginbas-Orakci, G., Dreisigacker, S., Imren, M., Toktay, H., Elekcioglu, H. I., Mekete, T., Nicol, J. M., Ansari, O., & Ogbonnaya, F. (2016). Association analysis of resistance to cereal cyst nematodes (Heterodera avenae) and root lesion nematodes (Pratylenchus neglectus and P. thornei) in CIMMYT advanced spring wheat lines for semi-arid conditions. Breeding Science, 66(5), 692–702.
de la Pena, E., Karssen, G., & Moens, M. (2007). Distribution and diversity of root-lesion nematodes (Pratylenchus spp.) associated with Ammophila arenaria in coastal dunes of Western Europe. Nematology, 9(6), 881–901.
De Luca, F., Reyes, A., Troccoli, A., & Castillo, P. (2011). Molecular variability and phylogenetic relationships among different species and populations of Pratylenchus (Nematoda: Pratylenchidae) as inferred from the analysis of the ITS rDNA. European Journal of Plant Pathology, 130(3), 415–426.
Gasser, R. B., & Newton, S. E. (2000). Genomic and genetic research on bursate nematodes: Significance, implications and prospects. International Journal for Parasitology, 30(4), 509–534.
Hu, M. X., Zhuo, K., & Liao, J. L. (2011). Multiplex PCR for the simultaneous identification and detection of Meloidogyne incognita, M. enterolobii, and M. javanica using DNA extracted directly from individual galls. Phytopathology, 101(11), 1270–1277.
Janssena, T., Karssen, G., Orlandoa, V., Subbotin, S. A., & Bert, W. (2017). Molecular characterization and species delimiting of plant-parasitic nematodes of the genus Pratylenchus from the penetrans group (Nematoda: Pratylenchidae). Molecular Phylogenetics and Evolution, 117, 30–48.
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., Jones, M. G. K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J. E., Wesemael, W. M. L., & Perry, R. N. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology, 14(9), 946–961.
Madani, M., Subbotin, S. A., & Moens, M. (2005). Quantitative detection of the potato cyst nematode, Globodera pallida, and the beet cyst nematode, Heterodera schachtii, using real-time PCR with SYBR green I dye. Molecular and Cellular Probes, 19(2), 81–86.
Mokrini, F., Waeyenberge, L., Viaene, N., Andaloussi, F. A., & Moens, M. (2013). Quantitative detection of the root-lesion nematode, Pratylenchus penetrans, using qPCR. European Journal of Plant Pathology, 137(2), 403–413.
Mokrini, F., Waeyenberge, L., Viaene, N., Andaloussi, F. A., & Moens, M. (2014). The beta-1,4-endoglucanase gene is suitable for the molecular quantification of the root-lesion nematode, Pratylenchus thornei. Nematology, 16, 789–796.
Powers, T. O., Todd, T. C., Burnell, A. M., Murray, P. C. B., Fleming, C. C., Szalanski, A. L., Adams, B. A., & Harris, T. S. (1997). The rDNA internal transcribed spacer region as a taxonomic marker for nematodes. Journal of Nematology, 29(4), 441–450.
Qiu, J., Westerdahl, B. B., & Williamson, V. M. (2007). Detection and quantification of root-lesion nematode Pratylenchus vulnus using realtime PCR. Journal of Nematology, 39(1), 95–96.
Saponari, M., Loconsole, G., Liao, H. H., Jiang, B., Savino, V., & Yokomi, R. K. (2013). Validation of high-throughput real time polymerase chain reaction assays for simultaneous detection of invasive citrus pathogens. [article]. Journal of Virological Methods, 193(2), 478–486.
Sato, E., Min, Y. Y., Toyota, K., & Takada, A. (2009). Relationships between the damage to radish caused by the root-lesion nematode Pratylenchus penetrans, its density prior to cultivation and the soil nematode community structure evaluated by polymerase chain reaction-denaturing gradient gel electrophoresis. Soil Science and Plant Nutrition, 55(4), 478–484.
Sato, E., Goto, K., Min, Y. Y., Toyota, K., & Suzuki, C. (2010). Quantitative detection of Pratylenchus penetrans from soil by using soil compaction and real-time PCR. Nematological Research, 40(1), 1–6.
Smiley, R. W., Yan, G., & Gourlie, J. A. (2014). Selected Pacific northwest rangeland and weed plants as hosts of Pratylenchus neglectus and P. thornei. Plant Disease, 98(10), 1333–1340.
Subbotin, S. A., Vierstraete, A., De Ley, P., Rowe, J., Waeyenberge, L., Moens, M., & Vanfleteren, J. R. (2001). Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Molecular Phylogenetics and Evolution, 21(1), 1–16.
Subbotin, S. A., Ragsdale, E. J., Mullens, T., Roberts, P. A., Mundo-Ocampo, M., & Baldwin, J. G. (2008). A phylogenetic framework for root lesion nematodes of the genus Pratylenchus (Nematoda): Evidence from 18S and D2-D3 expansion segments of 28S ribosomal RNA genes and morphological characters. Molecular Phylogenetics and Evolution, 48(2), 491–505.
Wang, H. H., Zhuo, K., Ye, W. M., & Liao, J. L. (2015). Morphological and molecular characterisation of Pratylenchus parazeae n. sp. (Nematoda: Pratylenchidae) parasitizing sugarcane in China. European Journal of Plant Pathology, 143(1), 173–191.
Yan, G., Smiley, R. W., Okubara, P. A., Skantar, A., Easley, S. A., Sheedy, J. G., & Thompson, A. L. (2008). Detection and discrimination of Pratylenchus neglectus and P. thornei in DNA extracts from soil. Plant Disease, 92(11), 1480–1487.
Yan, G., Smiley, R. W., & Okubara, P. A. (2012). Detection and quantification of Pratylenchus thornei in DNA extracted from soil using real-time PCR. Phytopathology, 102(1), 14–22.
Yan, G., Smiley, R. W., Okubara, P. A., Skantar, A. M., & Reardon, C. L. (2013). Developing a real-time PCR assay for detection and quantification of Pratylenchus neglectus in soil. Plant Disease, 97(6), 757–764.
Acknowledgements
This work was supported by the Planning Project for Forestry Science and Technology in Guangdong Province (grant number 2015KJCX045) and the Planning Project for Science and Technology in Guangzhou City (grant number 11A62100574).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human participants
The research does not involve human participants or any protected and/or endangered species.
Conflict of interest
There are no conflicts of interest for this work.
Consent of publication
All authors agree on the publication of the manuscript.
Rights and permissions
About this article
Cite this article
Lin, B., Tao, Y., Wang, H. et al. Duplex real-time quantitative PCR for simultaneous detection and quantification of Pratylenchus neglectus and P. thornei. Eur J Plant Pathol 157, 185–196 (2020). https://doi.org/10.1007/s10658-020-01999-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-01999-7