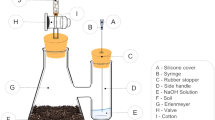
Abstract
Endosulfan, 6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano,2,4,3-benzodioxathiepin-3-oxide, is still a pesticide of choice for most cocoa farmers in Southwestern Nigeria, in spite of its persistence, bioaccumulative, toxicological properties, and restriction. A single treatment of 1.4 kg ai/ha (0.5% ai) of technical grade endosulfan (Thiodan, 35EC) was applied to 0.0227 ha of cultivated Theobroma cacao L. (Cocoa) farm at the Cocoa Research Institute of Nigeria (CRIN). Levels of parent endosulfan (α-, β-endosulfan) and major metabolite (endosulfan sulfate) were determined in vegetation and surrounding matrices at days 0, 7, 14, 21, 28, 42, and 60 using GC-MS. Their kinetic variables were determined. Order of ∑endosulfan distribution at day 0 was dry foliage > fresh foliage > bark > pods > soil (0–15 cm). No residual endosulfan was found in cocoa seeds and subsurface soil (15–30 cm). Low residual levels in pods on day 0 may be due to endogenous enzymatic breakdown, with α-isomer more susceptible and α/β-endosulfan ratio being 0.90. Fell dry foliage as mulch was predominantly the receiving matrix for non-target endosulfan sprayed. Volatilization was key in endosulfan dissipation between days 0 and 7 from foliage surfaces (> 60% loss), while dissipation trend was bi-phasic and tri-phasic for vegetation and soil, respectively. ∑endosulfan loss at terminal day ranged between 40.60% (topsoil) and 99.47% (fresh foliage). Iteratively computed half-lives (DT′50) ranged from 6.48 to 30.13 days for ∑endosulfan in vegetation. Endosulfan was moderately persistent in pods—a potential source for cross contamination of seeds during harvest. Iteratively determined DT′50 and initial-final day DT50 are highly correlated (R = 0.9525; n = 28) and no significant difference (P = 0.05) for both methods.




Similar content being viewed by others
References
Aikpokpodion, P. E., Lajide, L., Ogunlade, M. O., Ipinmoroti, R., Orisajo, S., Iloyanomon, C. I., & Fademi, O. (2010). Degradation and residual effects of endosulfan on soil chemical properties and root- knot nematode Meloidogyne incognita populations in cocoa plantation in Ibadan, Nigeria. Journal of Applied Biosciences, 26, 1640–1646.
Aikpokpodion, P., Lajide, L., Aiyesanmi, A. F., Lacorte, S., Grimalt, J., & Carretero, J. (2012). Residues of diazinon and endosulfan in cocoa beans from three cocoa ecological zones in Nigeria. European Journal of Applied Sciences, 4(6), 265–272. https://doi.org/10.5829/idosi.ejas.2012.4.6.1787.
Antonious, G. F., Byers, M. E., & Snyder-Conn, E. (1998). Residues and fate of endosulfan on field grown pepper and tomato. Pesticide Science., 54, 61–67.
Australian Quarantine and Inspection Service AQIS. (2003). Pesticide risk profile for the grazing of pulse/legume forage/straw and/or cutting of hay and feeding to cattle and sheep (Prepared by Dugald MacLachlan), Department of Agriculture, Fisheries and Forestry. Canberra ACT 2601. www.aqis.gov.au. Assessed 29.01.19.
Babatola, E. B. (2013). Urban influence on relative-humidity and its corresponding effects on rainfall, a case study of Ibadan, Nigeria. Mediterranean Journal of Social Sciences, 4(4), 343–351. https://doi.org/10.5901/mjss.2013.v4n4p343.
Bateman, R. (2003). Rational pesticide use; spatially and temporally targeted application of specific products. In M. F. Wilson (Ed.), Optimizing pesticide use. Hoboken: Publisher Wiley.
Bateman, R. (2008). International Cocoa Organization (ICCO); a guide to pesticide use in cocoa. http://www.icco.org. Assessed 12.07.2014.
Beard, E. J., & Ware, W. G. (1969). Fate of endosulfan on plants and glass. Journal of Agriculture and Food Chemistry., 17, 216–220. https://doi.org/10.1021/jf60162a033.
Bohm, D. A., Stachel, C. S., & Gowik, P. (2010). Confirmatory method for the determination of streptomycin in apples by LC-MS/MS. Analytica Chimica Acta., 672(1-2), 103–106. https://doi.org/10.1016/j.aca.2010.03.056
Calamari, D., Bacci, E., Focardi, S., Gaggi, C., Morosini, M., & Vighi, M. (1991). Role of plant biomass in the global partitioning of chlorinated hydrocarbons. Environmental Science & Technology., 25, 1489–1495. https://doi.org/10.1021/es00020a020.
Camacho-Morales, L. R., & Sánchez, E. J. (2016). Chapter: 12 Biotechnological use of fungi for the degradation of recalcitrant agro-pesticides. In Firs (Ed.), Book: Mushroom biotechnology: Developments and applications (pp. 203–214). Cambridge: Publisher: Academic Press, Elsevier. https://doi.org/10.1016/B978-0-12-802794-3.00012-6.
CCME, (2010). Scientific criteria document for the development of the Canadian water quality guidelines for endosulfan. http://ceqg-rcqe.ccme.ca/download. Assessed 19.06.2015.
Ciglasch, H., Busche, J., Amelung, W., Totrakool, S., & Kaupenjohann, M. (2006). Insecticide dissipation after repeated field application to a Northern Thailand ultisol. Journal of Agriculture & Food Chemistry., 54(22), 8551–8559. https://doi.org/10.1021/jf061521u.
Daud, Z., Kassim, A. S. M., Aripin, A. M., Awang, H., Hatta, M., & Z. M. (2013). Chemical composition and morphological of cocoa pod husks and cassava peels for pulp and paper production. Australian Journal of Basic Applied Science, 7(9), 406–411.
European Commission. (2011). European Union database (PLANTS). Applicable from 21/10/2011. Reg. (EU) No 310/2011. http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.CurrentMRL&language=EN. Assessed 25.01.2019.
EXTOXNET, (1993). Movement of pesticides in the environment. Extension Toxicology Network, Oregon State University, USA. http://extoxnet.orst.edu. Assessed 09.09.2015.
Falade, O. A., Nwodo, U. U., Iweriebor, C. B., Ezekiel Green, E., Mabinya, V. L., & Okoh, I. A. (2016). Lignin peroxidase functionalities and prospective applications. A review: MicrobiologyOpen, 6(1). https://doi.org/10.1002/mbo3.394.
Fantke, P., & Juraske, R. (2013). Variability of pesticide dissipation half-lives in plants. Environmental Science and Technology, 47(8), 3548–3562. https://doi.org/10.1021/es303525x.
Fantke, P., Wieland, P., Wannaz, C., Friedrich, R., & Jolliet, O. (2013). Dynamics of pesticide uptake into plants: From system functioning to parsimonious modeling. Environmental Modelling & Software, 40, 316–324. https://doi.org/10.1016/j.envsoft.2012.09.016.
Fantke, P., Gillespie, B. W., Juraske, R., & Jolliet, O. (2014). Estimating half-lives for pesticide dissipation from plants. Environmental Science and Technology., 48, 8588–8602. dx.doi.org. https://doi.org/10.1021/es500434p.
FAO/WHO. (1989). Joint Committee, FAO/WHO (JCFW), Food Standards Programme, Codex Alimentarius Commission. In The report of the 20th Session of the Codex Committee on Pesticide Residues (CCPR). (Ref. ALINORM 89/24). Eighteenth Session, Geneva, 3–14 July 1989. Italy: FAO, 00100 Rome.
Food and Agriculture Organization (2006). Endosulfan evaluation. (First draft prepared by Bernard Declerq, Epinary sur Orge, France). http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation06/Endosulfan06.pdf (Accessed 22.01.2019).
Gelman, F., Binstock, R., & Halicz, L., (2011). Application of the Walkley-Black titration for organic carbon quantification in organic rich sedimentary rocks. The Ministry of National Infrastructures, Geological Survey of Israel. Report GSI/13/ 2011.
GFEA (German Federal Environment Agency) (2007). Endosulfan – LRTAP Protocol on POPs. http://www.unece.org/fileadmin/DAM/env/lrtap/TaskForce/popsxg/2008/endosulfan. Assessed 27.12.2018.
Ghadiri, H., & Rose, C. W. (2001). Degradation of endosulfan in a clay soil from cotton farms of western Queensland. Journal of Environmental Management, 62, 155–169.
Ghadiri, H., Rose, C. W., & Connell, D. W. (1995). Degradation of organochlorine pesticides in soils under controlled environment and outdoor conditions. Journal of Environmental Management, 43, 141–151. https://doi.org/10.1016/S0301-4797(95)90123-X.
Halsall, C. J. (2004). Investigating the occurrence of persistent organic pollutants (POPs) in the Arctic: Their atmospheric behaviour and interaction with the seasonal snow pack. Environmental Pollution, 128, 163–175. https://doi.org/10.1016/j.envpol.2003.08.026.
Hapeman, C. J., Schmidt, W. G., & Rice, C. P. (1997). Structural and thermodynamic considerations in the isomeric conversion of endosulfan. Proceedings of the 213th National Meeting of the American Chemical Society, 213(1-3) San Francisco, CA, USA, April 13-17.
ISO (2001). ISO 9276-2:2001: Representation of results of particle size analysis—Part 2: Calculation of average particle sizes/diameters and moments from particle size distributions.
Jacobsen, R. E., Fantke, P., & Trapp, S. (2015). Analysing half-lives for pesticide dissipation in plants. SAR and QSAR in Environmental Research., 26(4), 325–342. https://doi.org/10.1080/1062936X.2015.1034772.
Juraske, R., Anto’n, A., & Castells, F. (2008). Estimating half-lives of pesticides in/on vegetation for use in multimedia fate and exposure models. Chemosphere, 70, 1748–1755. https://doi.org/10.1016/j.chemosphere.2007.08.047.
Kameshwar, S. K. A., & Qin, W. (2018). Structural and functional properties of pectin and lignin–carbohydrate complexes de-esterases: A review. Bioresoures & Bioprocessing, 5, 43. https://doi.org/10.1186/s40643-018-0230-8.
Katagi, T. (2004). Photodegradation of pesticides on plant and soil surfaces. Reviews of Environmental Contamination & Toxicology., 182, 1–195.
Kathpal, T. S., Singh, A., Dhankhar, J. S., & Singh, G. (1997). Fate of endosulfan in cotton soil under subtropical conditions of northern India. Pesticide Science, 50, 21–27. https://doi.org/10.1002/(SICI)1096-9063(199705)50:1<21.
Kennedy, I. R., Sánchez-Bayo, F., Kimber, S. W., Hugo, L., & Ahmad, N. (2001). Off-site movement of endosulfan from irrigated cotton in New South Wales. Journal of Environmental Quality, 30, 683–696. https://doi.org/10.2134/jeq2001.303683x.
Kerle, E. A., Jenkins, J. J., & Vogue, P. A. (2007). Understanding pesticide persistence and mobility for groundwater and surface water protection. Oregon State University Extension Service, EM8561-E.
Kwon, G. S., Kim, J. E., Kim, T. K., Sohn, H. Y., Koh, S. C., Shin, K. S., & Kim, D. G. (2002). Klebsiella pneumoniae KE-1 degrades endosulfan without formation of the toxic metabolite, endosulfan sulfate. FEMS Microbiology Letters, 215, 255–259. https://doi.org/10.1111/j.1574-6968.2002.tb11399.x.
Mackay, D., Shui, W., & Ma, K. (1997). Illustrated handbook of physical-chemical properties and environmental fate of organic chemicals (pp. 351–374). Boca Raton: Lewis Publishers.
Malik, A., Ojha, P., & Singh, K. P. (2009). Levels and distribution of persistent organochlorine pesticide residues in water and sediments of Gomti River (India)—A tributary of the Ganges River. Environmental Monitoring Assessment, 148, 421–435. https://doi.org/10.1007/s10661-008-0172-2.
Miglioranza, K. S. B., Aizpún de Moreno, J. E., Moreno, V. J., Osterrieth, M. L., & Ėscalante, A. H. (1999). Fate of organochlorine pesticides in soils and terrestrial biota of “Los Padres” pond watershed, Argentina. Environmental Pollution, 105, 91–99. https://doi.org/10.1016/S0269-7491(98)00200-0.
Mukherjee, I., & Gopal, M. (1994). Interconversion of stereoisomers of endosulfan on chickpea crop under field conditions. Pesticide Science, 40, 103–106.
NAFTA/USEPA. (2006). Guidance document for conducting terrestrial field dissipation studies. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/nafta-guidance-document-conducting-terrestrial-field. (Assessed, 12.10.14).
National Research Council Canada (NRC). (1975). Endosulfan: Its effects on environmental quality. Subcommittee on Pesticides and Related Compounds, NRC Associate Committee on Scientific Criteria for Environ Quality, Report No. 11, Ottawa, Canada.
Ntow, W. J., Ameyibor, J., Kelderman, P., Drechsel, P., & Gijzen, H. J. (2007). Dissipation of endosulfan in field-grown tomato (Lycopersicon esculentum) and cropped soil at Akumadan, Ghana. Journal of Agriculture & Food Chemistry, 55(26), 10864–10871. https://doi.org/10.1021/jf0718648.
OECD (Organization for Economic Co-operation and Development). (2014). Harmonized International Guidance for Pesticide Terrestrial Field Dissipation Studies and Crosswalk of North American & European Eco-Regions, Part I Guidance for Conducting Pesticide Terrestrial Field Dissipation (Draft Document).
Ortiz-Hernández, M. L., Sánchez-Salinas, E., Dantán-González, E., & Castrejón-Godínez, M. L. (2013). Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In: Rolando Chamy (Ed.), Biodegradation - Life of Science, Chapter 1, (pp 251–287). https://doi.org/10.5772/56098
Osibanjo, O. (2003). Organochlorine in Nigeria and Africa; The handbook of environmental chemistry, Vol. 3, part O, Persistent Organic Pollutants. Berlin Heideberg: Springer-Verlag.
Oyekunle, J. A. O., Akindolani, O. A., Sosan, M. B., & Adekunle, A. S. (2017). Organochlorine pesticide residues in dried cocoa beans obtained from cocoa stores at Ondo and Ile-Ife, Southwestern Nigeria. Toxicological Reports, 4, 151–159. https://doi.org/10.1016/j.toxrep.2017.03.001.
Padi, B., & Owusu, G. K. (1998). Towards an integrated pest management for sustainable cocoa production in Ghana. National Zoological Park. http://nationalzoo.si.edu/scbi/migratorybirds/research/cacao/padi. Assessed 07.06.2010.
Raha, P., Banerjee, H., Das, A. K., & Adityachaudhury, N. (1993). Persistence kinetics of endosulfan, fenvalerate and decamethrin in and on eggplant (Solanum melongena L.). Journal of Agriculture & Food Chemistry., 41, 923–928. https://doi.org/10.1021/jf00030a017.
Reischl, A., Reissinger, M., Thoma, H., & Hutzinger, O. (1989). Accumulation of organic air constituents by plant surface: Part IV. Chemosphere, 18, 561–568. https://doi.org/10.1016/0045-6535(89)90167-7.
Reynolds, G. S. (1970). The gravimetric method of soil moisture determination. Journal of Hydrology, 11, 258–273.
Rice, C. P., Happeman, C. J., & Chernyak, S. M. (1997). Experimental evidence for the interconversion of endosulfan isomers, Proceedings of the 213 th National Meeting of the American Chemical Society, vol. 213 (1-3) San Francisco, CA, USA, April 13-17.
Rice, C., Nochetto, C., & Zara, P. (2002). Volatilization of trifluralin, atrazine, metolachlor, chlorpyrifos, α-endosulfan and β-endosulfan from freshly tilled soil. Journal of Agriculture & Food Chemistry, 50, 4009–4017. https://doi.org/10.1021/jf011571t.
Rosendahl, I., Laabs, V., Atcha-Ahowe, C., Braima, J., & Amelung, W. (2009). Insecticide dissipation from soil and plant surfaces in tropical horticulture of southern Benin, West Africa. Journal of Environmental Monitoring, 11, 1157–1164. https://doi.org/10.1039/B903470F.
Schmidt, W. F., Bilboulian, S., Rice, C. P., Fettinger, J. C., McConnell, L. L., & Hapeman, C. J. (2001). Thermodynamic, spectroscopic and computational evidence for the irreversible conversion of beta- to alpha-endosulfan. Journal of Agriculture & Food Chemistry, 49, 5372–5376. https://doi.org/10.1021/jf0102214.
Sethunathan, N., Megharaj, M., Chen, Z. L., Williams, B. D., Lewis, G., & Naidu, R. (2004). Algal degradation of a known endocrine disrupting insecticide, alpha-endosulfan, and its metabolite, endosulfan sulphate, in liquid medium and soil. Journal of Agriculture & Food Chemistry., 52, 3030–3035. https://doi.org/10.1021/jf035173x.
Shrivastava, A., & Gupta, V. B. (2011). Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles of Young Scientist, 2, 21–25
Siddique, T., Okeke, B. C., Arshad, A., & Frankenberger, W. T., Jr. (2003). Enrichment and isolation of endosulfan degrading microorganisms. Journal Environmental Quality., 32, 47–54.
Stein, S. E. (1995). Chemical substructure identification by mass spectral library searching. Journal of the American Society for Mass Spectrometry, 6(8), 644–655. https://doi.org/10.1016/S1044-0305(05)80054-6.
Thomas, G. W. (1982). Exchangeable cations: In Methods of soil analysis Part 2, Chemical and microbiological methods, Page A.L., (Ed.) Agronomy monograph No.9 (2), 159-166.
Tiwari, M. K., & Guha, S. (2013). Kinetic of the biodegradation pathway of endosulfan in the aerobic and anaerobic environments. Chemosphere, 93, 567–573. https://doi.org/10.1016/j.chemosphere.2013.07.005.
Tomlin, C. D. S. (Ed.). (2000). The pesticide manual (12th ed.p. 821). London: Crop Protection Publications.
Trapp, S., & Legind, C. N. (2011). Uptake of organic contaminants from soil into vegetables and fruits. In F. A. Swartjes (Ed.), Dealing with Contaminated Sites From Theory towards Practical Application (pp. 369–408). Dordrecht, The Netherlands: Springer Press.
UNEP (1999). ENDOSULFAN-21-VOL3_B8_.DOC http://chm.pops.int/Portals/0/Repository/Endosulfan2008/UNEP-POPS-POPRC-END-08-EU-V3-8.English.PDF. Assessed 27.12.2018.
UNEP. (2009). Stockholm convention on persistent organic pollutants. Supporting documents for the draft risk profile on endosulfan. POPs Review Committee, Fifth meeting (12-16 October, 2009), Geneva. UNEP/POPs/POPRC.5/INF/9.
UNEP, (2011). Stockholm convention on persistent organic pollutants. UNEP-POPS-Treaty-Notif-CN703-2011 on endosulfan. http://chm.pops.int/. (Assessed 10.03.2016).
USEPA, (2002a). Method 3570—Microscale solvent extraction (MSE) of volatile, semivolatile and nonvolatile organic compound from solids.
USEPA, 2002b. Endosulfan RED facts. USEPA, Office of Prevention, Pesticides. http://www.epa.gov/oppsrrd1/REDs/factsheets/endosulfan_fs.htm. (Assessed 10.09.2018).
Vaikosen, N. E., Gibson, T. L., Davidson, M. C., Olu-Owolabil, I. B., Adebowale, O. K., Ebeshi, U. B., & Diagboya, E. N. P. (2018). GC-MS fragmentation patterns of sprayed endosulfan and its sulphate metabolite in samples of Theobroma cacao L from a field kinetic study. European Journal of Mass Spectrometry. https://doi.org/10.1177/1469066718817690.
Walkley, A., & Black, A. I. (1934). An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science., 37, 29–37.
Walse, S. S., Scott, G. I., & Ferry, J. L. (2003). Stereo-selective degradation of aqueous endosulfan in modular estuarine mesocosms: Formation of endosulfan γ-hydroxycarboxylate. Journal of Environmental Monitoring, 5, 373–379. https://doi.org/10.1039/B212165D.
Wauchope, R. D., Johnson, W. C., & Sumner, H. R. (2004). Foliar and soil deposition of pesticide sprays in peanuts and their wash off and runoff under stimulated worst-case rainfall. Journal of Agriculture & Food Chemistry, 52, 7056–7063. https://doi.org/10.1021/jf049283v.
Weber, J., Halsall, J. C., Muir, D., Teixeira, C., Small, J., Solomon, K., Hermanson, M., Hung, H., & Bidleman, T. (2010). Endosulfan, a global pesticide: A review of its fate in the environment and occurrence in the Arctic. Science of Total Environment, 408, 2966–2984. https://doi.org/10.1016/j.scitotenv.2009.10.077.
Weir, K. M., Sutherland, T. D., Horne, I., Russell, R. J., & Oakeshott, J. G. (2006). A single monooxygenase, ese, is involved in the metabolism of the organochlorides endosulfan and endosulfate in an Arthrobacter sp. Applied Environmental Microbiology, 72(5), 3524–3530. https://doi.org/10.1128/AEM.72.5.3524-3530.2006.
Wolejko, E., Kaczynski, P., Lozowicka, B., Wydro, U., Andrzej, B., Hrynko, I., Konecki, R., Snarska, K., Dec, D., & Malinowski, P. (2017). Dissipation of S-metolachlor in plant and soil and effect on enzymatic activities. Environmental Monitoring & Assessment, 189(355), 1–16. https://doi.org/10.1007/s10661-017-6071-7
Wood, G. A. R., & Lass, R. A. (2011). Cocoa, 4th edition. Oxford UK: Published by John Wiley and Sons Ltd., Imprint - Blackwell Science.
Yeboah, P. O., Lowor, S., & Akpabli, C. K. (2003). Comparison of thin layer chromatography (TLC) and gas chromatography determination of propoxur residues in a cocoa ecosystem. African Journal Science & Technology Science & Engineering. Series, 4(2), 24–28.
Yu, Q., Qin, S., Wang, X., & Qiao, X. (2006). Dissipation of acetamiprid and imidacloprid under different temperature, light and biological factors on phyllosphere of Brassica chinensis. Chinese Journal of Pesticide Science, 8, 147–151.
Acknowledgements
We sincerely acknowledge the Tertiary Education Trust Funds (TETFUND) for funding part of this research through the Niger Delta University and also the University of Strathclyde, Glasgow, UK, for the study fellowship and full access to their research laboratories granted to Vaikosen. E. N.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vaikosen, E.N., Olu-Owolabi, B.I., Gibson, L.T. et al. Kinetic field dissipation and fate of endosulfan after application on Theobroma cacao farm in tropical Southwestern Nigeria. Environ Monit Assess 191, 196 (2019). https://doi.org/10.1007/s10661-019-7293-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7293-7