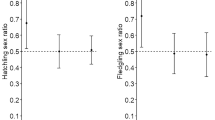
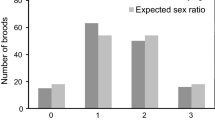
Abstract
Sex allocation studies among birds and mammals are notoriously inconsistent with theoretical predictions. One explanation is the difficulty of collecting data on costs and benefits of sex-ratio adjustments, which prevents the investigation of underlying assumptions. Some predictions may thus have been tested in species where they should not have been expected. Here, we focus on the “cost of reproduction hypothesis”, which states that parents with low investment capacity should avoid producing the most expensive sex to minimise the decrease in their residual reproductive value. In the black-legged kittiwake (Rissa tridactyla), sons are energetically more expensive than daughters. Using 10 years of data (1172 chicks from 790 broods) from a long-term feeding experiment, we predicted a stronger decrease in the probability of producing a son with deteriorating environmental conditions among Control than among supplementally Fed parents. To test this prediction, we used three proxies of environmental conditions and a recent sliding window approach. We found no support for our prediction. Hence, we investigated between-year sex-ratio variation in relation to feeding status to detect a response to an unmeasured environmental variable. There was no interaction between year and feeding status, nor any effect of feeding status itself. However, the probability of producing a male increased with time, which could be a response to an oceanic regime shift that occurred around our colony, but that our proxies failed to capture. Our study further highlights the difficulty of explaining sex-ratio variation in long-lived species with complex life-histories where multiple selective pressures can occur simultaneously.

Similar content being viewed by others
Data archiving
The data and R code are archived in the Open Science Framework repository (https://doi.org/10.17605/osf.io/gfpy8; https://osf.io/gfpy8/).
References
Anderson DJ, Reeve J, Gomez JEM et al (1993) Sexual size dimorphism and food requirements of nestling birds. Can J Zool 71:2541–2545
Arnbom T, Fedak MA, Rothery P (1994) Offspring sex ratio in relation to female size in southern elephant seals, Mirounga leonina. Behav Ecol Sociobiol 35:373–378
Bailey LD, van de Pol M (2016) climwin: an R toolbox for climate window analysis. PLoS ONE 11:e0167980
Bartoń K (2016) MuMIn: multi-model inference (version 1.15.6). https://CRAN.R-project.org/package=MuMIn. Accessed 17 Dec 2018
Bates D, Maechler M, Bolker BM (2011) package “lme4”: linear mixed-effects models using S4 classes (version 0.999375-42). http://cran.r-project.org/web/packages/lme4/index.html. Accessed 17 Dec 2018
Bérubé CH, Festa-Bianchet M, Jorgenson JT (1996) Reproductive costs of sons and daughters in Rocky Mountain bighorn sheep. Behav Ecol 7:60–68
Blanchard P, Festa-Bianchet M, Gaillard JM, Jorgenson JT (2005) Maternal condition and offspring sex ratio in polygynous ungulates: a case study of bighorn sheep. Behav Ecol 16:274–279
Bowers EK, Thompson CF, Sakaluk SK (2015) Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol 84:473–486
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, Berlin
Cameron EZ (2004) Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc R Soc London Ser B Biol Sci 271:1723–1728
Charnov E (1982) The theory of sex allocation. Princeton University Press, Princeton
Clutton-Brock T, Sheldon BC (2010) Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol Evol 25:562–573
Cockburn A, Double MC (2008) Cooperatively breeding superb fairy-wrens show no facultative manipulation of offspring sex ratio despite plausible benefits. Behav Ecol Sociobiol 62:681–688
Cockburn A, Legge S, Double M (2002) Sex ratios in birds and mammals: can the hypotheses be disentangled. In: Hardy ICW (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge, pp 266–286
Coulson JC (2011) The kittiwake. T. & A.D Poyser, London
Desfor KB, Boomsma JJ, Sunde P (2007) Tawny Owls Strix aluco with reliable food supply produce male-biased broods. Ibis 149:98–105
Desprez M, Jenouvrier S, Barbraud C et al (2018) Linking oceanographic conditions, migratory schedules and foraging behaviour during the non-breeding season to reproductive performance in a long-lived seabird. Funct Ecol. https://doi.org/10.1111/1365-2435.13117
Douhard M (2017) Offspring sex ratio in mammals and the Trivers-–Willard hypothesis: in pursuit of unambiguous evidence. BioEssays 39:1700043
Douhard M, Festa-Bianchet M, Coltman DW, Pelletier F (2016a) Paternal reproductive success drives sex allocation in a wild mammal. Evolution 70:358–368
Douhard M, Festa-Bianchet M, Pelletier F (2016b) Maternal condition and previous reproduction interact to affect offspring sex in a wild mammal. Biol Lett 12:20160510
Edwards AM, Cameron EZ, Pereira JC et al (2016) Gestational experience alters sex allocation in the subsequent generation. R Soc Open Sci 3:160210
Festa-Bianchet M (1996) Offspring sex ratio studies of mammals: does publication depend upon the quality of the research or the direction of the results? Ecoscience 3:42–44
Frank SA (1990) Sex allocationn theory for birds and mammals. Annu Rev Ecol Syst 21:13–55
Frederiksen M, Edwards M, Mavor RA, Wanless S (2007) Regional and annual variation in black-legged kittiwake breeding productivity is related to sea surface temperature. Mar Ecol Prog Ser 350:137–143
Gelman A, Su Y-S (2014) arm: data analysis using regression and multilevel/hierarchical models. R package version 1.7-03. http://CRAN.R-project.org/package=arm. Accessed 17 Dec 2018
Gill VA, Hatch SA (2002) Components of productivity in black-legged kittiwakes Rissa tridactyla: response to supplemental feeding. J Avian Biol 33:113–126
Gomendio M, Clutton-Brock TH, Albon SD et al (1990) Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343:261–263
Grecian WJ, Witt MJ, Attrill MJ et al (2016) Seabird diversity hotspot linked to ocean productivity in the Canary Current Large Marine Ecosystem. Biol Lett 12:20160024
Hardy I (2002) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge
Hatch SA (2013) Kittiwake diets and chick production signal a 2008 regime shift in the Northeast Pacific. Mar Ecol Prog Ser 477:271–284
Hatch SA, Robertson GJ, Baird HP (2009) Black-legged kittiwake (Rissa tridactyla). The birds of North America online. Cornell Laboratory of Ornithology, Ithaca
Helfenstein F, Danchin E, Wagner RH (2004) Assortative mating and sexual size dimorphism in black-legged Kittiwakes. Waterbirds 27:350–354
Hewison AJM, Gaillard JM (1999) Successful sons or advantaged daughters? The Trivers–Willard model and sex-biased maternal investment in ungulates. Trends Ecol Evol 14:229–234
Hörnfeldt B, Hipkiss T, Fridolfsson A-K et al (2000) Sex ratio and fledging success of supplementary-fed Tengmalm’s owl broods. Mol Ecol 9:187–192
Jodice PGR, Lanctot RB, Gill VA et al (2000) Sexing adult black-legged kittiwakes by DNA, behavior, and morphology. Waterbirds 23:405–415
Kalmbach E, Nager RG, Griffiths R, Furness RW (2001) Increased reproductive effort results in male-biased offspring sex ratio: an experimental study in a species with reversed sexual size dimorphism. Proc R Soc London Ser B Biol Sci 268:2175–2179
Keogan K, Daunt F, Wanless S et al (2018) Global phenological insensitivity to shifting ocean temperatures among seabirds. Nat Clim Chang 8:313
Khwaja N, Preston SA, Briskie JV, Hatchwell BJ (2018) Testing the predictions of sex allocation hypotheses in dimorphic, cooperatively breeding riflemen. Ecol Evol 8:3693–3701
Komdeur J (2012) Sex allocation. In: Royle Nick J, Smiseth Per T, Kölliker Mathias (eds) The evolution of parental care. Oxford University Press, Oxford, pp 171–188
Komdeur J, Pen I (2002) Adaptive sex allocation in birds: the complexities of linking theory and practice. Philos Trans R Soc London Ser B Biol Sci 357:373–380
Komdeur J, Daan S, Tinbergen J, Mateman C (1997) Extreme adaptive modification in sex ratio of the Seychelles warbler’s eggs. Nature 385:522–525
Kotzerka J, Garthe S, Hatch SA (2010) GPS tracking devices reveal foraging strategies of Black-legged Kittiwakes. J Ornithol 151:459–467
Krackow S, Neuhäuser M (2008) Insights from complete-incomplete brood sex-ratio disparity. Behav Ecol Sociobiol 62:469–477
Krijgsveld KL, Daan S, Dijkstra C, Visser GH (1998) Energy requirements for growth in relation to sexual size dimorphism in marsh harrier Circus aeruginosus nestlings. Physiol Biochem Zool 71:693–702
Kruuk LE, Osmond HL, Cockburn A (2015) Contrasting effects of climate on juvenile body size in a Southern Hemisphere passerine bird. Glob Chang Biol 21:2929–2941
Langmore NE, Bailey LD, Heinsohn RG et al (2016) Egg size investment in superb fairy-wrens: helper effects are modulated by climate. Proc R Soc B 283:20161875
Magrath MJL, van Lieshout E, Pen I et al (2007) Estimating expenditure on male and female offspring in a sexually size-dimorphic bird: a comparison of different methods. J Anim Ecol 76:1169–1180
Malo AF, Martinez-Pastor F, Garcia-Gonzalez F et al (2017) A father effect explains sex-ratio bias. Proc R Soc B 284:20171159. https://doi.org/10.1098/rspb.2017.1159
Martin JG, Festa-Bianchet M (2011) Sex ratio bias and reproductive strategies: what sex to produce when? Ecology 92:441–449
Mazerolle MJ (2013) AICcmodavg: model selection and multimodel inference based on (Q) AIC (c). In: R package version 1.35. http://CRAN.R-project.org/package=AICcmodavg. Accessed 17 Dec 2018
McDonald PG, Olsen PD, Cockburn A (2005) Sex allocation and nestling survival in a dimorphic raptor: does size matter? Behav Ecol 16:922–930
Merkling T, Leclaire S, Danchin E et al (2012) Food availability and offspring sex in a monogamous seabird: insights from an experimental approach. Behav Ecol 23:751–758
Merkling T, Welcker J, Hewison AJM et al (2015) Identifying the selective pressures underlying offspring sex-ratio adjustments: a case study in a wild seabird. Behav Ecol 26:916–925
Merkling T, Blanchard P, Chastel O et al (2017) Reproductive effort and oxidative stress: effects of offspring sex and number on the physiological state of a long-lived bird. Funct Ecol 31:1201–1209
Merkling T, Nakagawa S, Lagisz M, Schwanz LE (2018) Maternal testosterone and offspring sex-ratio in birds and mammals: a meta-analysis. Evol Biol 45:96–104
Moreno-Rueda G, Campos F, Gutiérrez-Corchero F, Hernández M (2014) Costs of rearing and sex-ratio variation in southern grey shrike Lanius meridionalis broods. J Avian Biol 45:424–430
Myers JH (1978) Sex-ratio adjustment under food stress - maximization of quality or numbers of offspring. Am Nat 112:381–388
Nager RG, Monaghan P, Griffiths R et al (1999) Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci USA 96:570–573
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Navara KJ (2013) The role of steroid hormones in the adjustment of primary sex ratio in birds: compiling the pieces of the puzzle. Integr Comp Biol 53:923–937
Navara KJ (ed) (2018) Hormones rule the roost: hormonal influences on sex ratio adjustment in birds and mammals. In: Choosing sexes. Springer, Berlin, pp 123–154
Nichols HJ, Fullard K, Amos W (2014) Costly sons do not lead to adaptive sex ratio adjustment in pilot whales, Globicephala melas. Anim Behav 88:203–209
Pen I, Weissing FJ (2002) Optimal sex allocation: steps towards a mechanistic theory. In: Hardy ICW (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge
Post E, Forchhammer MC, Stenseth NC, Langvatn R (1999) Extrinsic modification of vertebrate sex ratios by climatic variation. Am Nat 154:194–204
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Schaper SV, Dawson A, Sharp PJ et al (2012) Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. Am Nat 179:E55–E69
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Schindler S, Gaillard J-M, Grüning A et al (2015) Sex-specific demography and generalization of the Trivers–Willard theory. Nature 526:249
Sheldon BC, West SA (2004) Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am Nat 163:40–54
Sydeman WJ, Thompson SA, Kitaysky A (2012) Seabirds and climate change: roadmap for the future. Mar Ecol Prog Ser 454:107–117
Torres R, Drummond H (1999) Does large size make daughters of the blue-footed booby more expensive than sons? J Anim Ecol 68:1133–1141
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary sex-ratio of offspring. Science 179:90–92
van de Pol M, Brouwer L, Brooker LC et al (2013) Problems with using large-scale oceanic climate indices to compare climatic sensitivities across populations and species. Ecography 36:249–255
van de Pol M, Bailey LD, McLean N et al (2016) Identifying the best climatic predictors in ecology and evolution. Methods Ecol Evol 7:1246–1257
Vedder O, Bouwhuis S, Benito MM, Becker PH (2016) Male-biased sex allocation in ageing parents; a longitudinal study in a long-lived seabird. Biol Lett 12:20160260
Vincenzi S, Hatch S, Mangel M, Kitaysky A (2013) Food availability affects onset of reproduction in a long-lived seabird. Proc R Soc B Biol Sci 280:20130554
Vincenzi S, Hatch S, Merkling T, Kitaysky AS (2015) Carry-over effects of food supplementation on recruitment and breeding performance of long-lived seabirds. Proc R Soc B 282:20150762
Weimerskirch H, Lallemand J, Martin J (2005) Population sex ratio variation in a monogamous long-lived bird, the wandering albatross. J Anim Ecol 74:285–291
Welcker J, Speakman JR, Elliott KH et al (2015) Resting and daily energy expenditures during reproduction are adjusted in opposite directions in free-living birds. Funct Ecol 29:250–258
West SA (2009) Sex allocation. Princeton University Press, Princeton
West SA, Sheldon BC (2002) Constraints in the evolution of sex ratio adjustment. Science 295:1685–1688
Wiebe KL, Bortolotti GR (1992) Facultative sex-ratio manipulation in American kestrels. Behav Ecol Sociobiol 30:379–386
Acknowledgements
Many volunteer and student field workers assisted in the field. We particularly thank the several camp leaders who supervised Middleton Island field work in one or more seasons: V. A. Gill, C. Sterne, N. A. Bargmann, A. M. Ramey, J. Kotzerka, T. van Nus, L. Agdere, K. Elliott and L. Chivers. We are grateful to the undergraduate students who helped with chick sexing. We also would like to thank Liam Bailey for his help with the climwin analyses, Nina McLean for helpful comments on a previous version and the Ocean Biology Processing Group at NASA’s Goddard Space Flight Centre for access to the chlorophyll-a data. We thank reviewers for their valuable comments on a previous version of this manuscript. Computations were performed on EDB-Calc Cluster which uses software developed by the Rocks(r) Cluster Group (San Diego Supercomputer Center, University of California, San Diego and its contributors), hosted by EDB. We thank P. Solbes for support. Field work was supported by the North Pacific Research Board (Project No. 320, BEST-BSIERP Projects B74, B67, and B77) to S.A.H. and by a Grant from the French Polar Institute Paul-Emile Victor (IPEV ‘Programme 1162 SexCoMonArc’) to P.B, S.L. and E.D. This work originated in the laboratory “Evolution et Diversité Biologique” (EDB) and was supported by the French Laboratory of Excellence Project “TULIP” (ANR-10-LABX-41; ANR-11-IDEX-0002-02). T.M. was supported by a French doctoral scholarship and a Fyssen post-doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All the work was conducted under the approval of the USGS Alaska Science Center Animal Care and Use Committee and the IPEV Ethical Committee, in accordance with United States laws and under permits from the U.S. Fish and Wildlife Service and the State of Alaska. Any use of trade names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Merkling, T., Hatch, S.A., Leclaire, S. et al. Offspring sex-ratio and environmental conditions in a seabird with sex-specific rearing costs: a long-term experimental approach. Evol Ecol 33, 417–433 (2019). https://doi.org/10.1007/s10682-019-09983-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-09983-2