Abstract
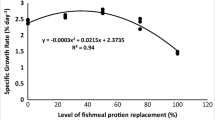
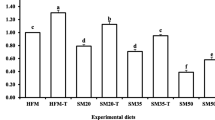
We evaluated the efficacy of mozuku fucoidan supplementation to alternative dietary proteins used in fish meal (FM) replacement to enhance growth, immunity, and stress resistance of Pagrus major. Seven isonitrogenous (45% protein) experimental diets were formulated where diet 1 (D1) was FM-based control diet. Diets 2 to 7 were formulated by replacing 25, 50, and 75% of FM protein with soy protein isolate (SPI) protein, and each replacement level was supplemented without or with fucoidan at 0.4% for diet groups D2 (FM25), D3 (FM25Fu), D4 (FM50), D5 (FM50Fu), D6 (FM75), and D7(FM75Fu), respectively. Each diet was randomly allocated to triplicate groups of fish (4.1 g) for 56 days. Significantly higher weight gain and specific growth rate were observed in fish fed FM50Fu diet group, and it was not differed (P > 0.05) with fish fed FM25Fu diet group. FM-based control diet showed intermediate value, and it was not differed (P > 0.05) with or without fucoidan-supplemented ≤ 50% FM replacement groups and FM75Fu diet group. Significantly lower growth performances were observed in FM75 diet group. At each replacement level, fucoidan-supplemented groups showed nonsignificant improvement of feed utilization performances. Fish fed fucoidan-supplemented diets showed best condition of oxidative and freshwater stress resistance. Lysozyme activity, NBT, and peroxidase activity showed higher (P > 0.05) values in fucoidan-supplemented groups compared with the non-supplemented groups. Catalase activity was significantly lower in FM75Fu diet group. Catalase activity is significantly influenced by the interaction effects of fucoidan and FM replacement level. In conclusion, fucoidan supplementation could increase the efficiency of utilizing SPI (≥ 75%) without any adverse effects on red sea bream performance.



Similar content being viewed by others
References
Anderson DP, Siwicki AK (1995) Basic hematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian Aquaculture II. Philippines, Fish Health Section, Asian Fisheries Society, Manila, pp 185–202
Anderson RL, Wolf W (1995) Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J Nutr 125:581S–588S
Armstrong D, Browne R (1994) The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol 366:43–58
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis, 16th edn. INTERNATIONAL, Arlington, VA
Baeverfjord G, Krogdahl Å (1996) Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: a comparison with the intestines of fasted fish. J. Fish Dis 19:375–387
Barnes ME, Brown ML, Rosentrater KA, Sewell JR (2012) An initial investigation replacing fish meal with a commercial fermented soybean meal product in the diets of juvenile rainbow trout. Open J Anim Sci 2:234–243
Bureau DP, Harris AM, Cho CY (1998) The effects of purified alcohol extracts from soy products on feed intake and growth of chinook salmon (Oncorhynchus tshawytscha) and rainbow trout (Oncorhynchus mykiss). Aquaculture 161:27–43
Buttle LG, Burrells AC, Good JE, Williams PD, Southgate PJ, Burrells C (2001) The binding of soybean agglutinin (SBA) to the intestinal epithelium of Atlantic salmon Salmo salar and rainbow trout, Oncorhynchus mykiss, fed high levels of soybean meal. Vet Immunol Immunopathol 80:237–244
Celi P, Sullivan M, Evans D (2010) The stability of the reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) tests on stored horse blood. Vet J 183:217–218
Chen W, Ai QH, Mai KS, Xu W, Liufu Z, Zhang W, Cai Y (2011) Effects of dietary soybean saponins on feed intake, growth performance, digestibility and intestinal structure in juvenile Japanese flounder (Paralichthys olivaceus). Aquaculture 318:95–100
Chen L, Madl RL, Vadlani PV, Li L, Wang W (2013) Value added products from soybean: Removal of anti-nutritional factors via bioprocessing. In: El-Shemy HA (ed) Soybean bio-active compounds, Chapter 8. In Tech, Rijeka, Croatia
Chotigeat W, Tongsupa S, Supamataya K, Phongdara A (2004) Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture 233:23–30
Chou RL, Her BY, Su MS, Hwang G, Wu YH, Chen HY (2004) Substituting fishmeal with soybean meal in diets of juvenile cobia (Rachycentron canadum). Aquaculture 229:325–333
Deng J, Mai K, Ai Q, Zhang W, Wang X, Xu W, Liufu Z (2006) Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 258:503–513
El-Boshy M, El-Ashram A, Risha E, Abdelhamid F, Zahran E, Gab-Alla A (2014) Dietary fucoidan enhance the non-specific immune response and disease resistance in African catfish, Clarias gariepinus, immune suppressed by cadmium chloride. Vet Immunol Immunopathol 162:168–173
Fuchs VI, Schmidt J, Slater MJ, Zentek J, Buck BH, Steinhagen D (2015) The effect of supplementation with polysaccharides, nucleotides, acidifiers and Bacillus strains in fish meal and soybean-based diets on growth performance in juvenile turbot (Scophthalmus maximus). Aquaculture 437:243–251
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta196: 143-152
Goth L, Meszaros I, Nemeth H (1982) Serum catalase enzyme activity in acute pancreatitis. Clin Chem 28:1999–2000
Goth L, Nemeth H, Meszaros I (1983) Serum catalase activity for detection of hemolytic diseases. Clin Chem 29:741–742
Goth L, Meszaros I, Nemeth H (1987) Serum catalase enzyme activity in liver diseases. Acta Biol Hung 38:287–290
Haroun-Bouhedja F, Ellouali M, Sinquin C, Boisson-Vidal C (2000) Relationship between sulfate groups and biological activities of fucans. Thromb Res 100:453–459
Hossain MS, Koshio S (2017) Dietary substitution of fishmeal by alternative protein with guanosine monophosphate supplementation influences growth, digestibility, blood chemistry profile, immunity and stress resistance of red sea bream, Pagrus major. Fish Physiol Biochem 43:1629–1644
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM, Ono S, Fujieda T (2016a) Comparison of the effects of inosine and inosine monophosphate on growth, immune response, stress resistance and gut morphology of juvenile red sea bream, Pagrus major. Aquaculture 458:64–74
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM (2016b) Dietary effects of adenosine monophosphate to enhance growth, digestibility, innate immune responses and stress resistance of juvenile red sea bream, Pagrus major. Fish and shellfish Immunol 56:523–533
Hossain MS, Kader MA, Dey T, Sony NM, Bulbul M, Koshio S (2017) Effect of high inclusion of rendered animal by-product ingredients on growth, digestibility and economic performances in climbing perch Anabas testudineus. Aquac Res 48:931–940
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM, Islam MJ, Maekawa M, Fujieda T (2018) Substitution of dietary fishmeal by soybean meal with inosine administration influences growth, digestibility, immunity, stress resistance and gut morphology of juvenile amberjack Seriola dumerili. Aquaculture 488:174–188
Huang XH, Zhou H, Zhang H (2006) The effect of Sargassum fusiforme polysaccharides extracts on vibriosis resistance and immune activity of the shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol 20:750–757
Huang X, Nie S, Xie M (2017) Interaction between gut immunity and polysaccharides. Crit Rev Food Sci Nutr 57:2943–2955
Jiang Z, Okimura T, Yokose T, Yamasaki Y, Yamaguchi K, Oda T (2010) Effects of sulfated fucan, ascophyllan, from the brown alga Ascophyllum nodosum on various cell lines: a comparative study on ascophyllan and fucoidan. J Biosci Bioeng 110:113–117
Kader MA, Koshio S, Ishikawa M, Yokoyama S, Bulbul M (2010) Supplemental effects of some crude ingredients in improving nutritive values of low fishmeal diets for red sea bream, Pagrus major. Aquaculture 308:136–144
Kaushik SJ, Cravedi JP, Lallès JP, Sumpter J, Fauconneau B, Laroche M (1995) Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 133:257–274
Kissil GW, Lupatsch I, Higgs DA, Hardy RW (2000) Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquac Res 31:595–601
Krogdahl A, Bakke-Mckellep AM, Baeverfjord G (2003) Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac Nutr 9:361–371
Kumar S, Sahu NP, Pal AK, Choudhury D, Yengkokpam S, Mukherjee SC (2005) Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in L. rohita juveniles. Fish Shellfish Immunol 19:331–344
Li P, Burr GS, Goff JB, Whiteman KW, Davis KB, Vega RR, Neill WH, Gatlin DM III (2005) A preliminary study on the effects of dietary supplementation of brewer’s yeast and nucleotides, singularly or in combination, on juvenile red drum (Sciaenops ocellatus). Aquac Res 36:1120–1127
Li SS, Wang QK, He YH, Ren DD, Zhang ZY (2013) The extraction of serum lipids reducing function of fucoidan from seaweed Costaria costata. J Dalian Ocean Univ 28(1):93–98
Liu Y, Jiang XL, Lu Q, Guan HS (2000) Effects of mannuronate polysaccharide on enzymes of Penaeus chinensis related with immune and hemolysis. J Fish China 24(6):549–553
Lusas EW, Riaz MN (1995) Soy protein products: processing and use. J Nutr 125:573S–580S
Lygren B, Sveier H, Hjeltnes B, Waagbo R (1999) Examination of the immunomodulatory properties and the effect on disease resistance of dietary bovine lactoferrin and vitamin C fed to Atlantic salmon (Salmo salar) for a short-term period. Fish Shellfish Immunol 9:95–107
Macfarlane GT, Steed H, Macfarlane S (2008) Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol 104:305–344
Mambrini M, Roem AJ, Cravèdi JP, Lallès JP, Kaushik SJ (1999) Effects of replacing fishmeal with soy protein concentrate and of DL-methionine supplementation in high-energy, extruded diets on the growth and nutrient utilization of rainbow trout, Oncorhynchus mykiss. J Anim Sci 77:2990–2999
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88
Médale F, Boujard T, Vallée F, Blanc D, Mambrini M, Roem A, Kaushik S (1998) Voluntary feed intake, nitrogen and phosphorus losses in rainbow trout Oncorhynchus mykiss fed increasing dietary levels of soy protein concentrate. Aquat Living Resour 11:239–246
Morganti P, Bruno C, Guarneri F, Cardillo A, Del Ciotto P, Valenzano F (2002) Role of topical and nutritional supplement to modify the oxidative stress. Int J Cosmet Sci 24(6):331–339
Nakagawa H, Umino T, Tasaka Y (1997) Usefulness of Ascophyllum meal as a feed additive for red sea bream, Pagrus major. Aquaculture 151:275–281
Okai Y, Higashi-Okai K, Ishizaka S, Ohtani K, Mastsui-Yuasa I, Yamashita U (1996) Possible immunodulating activities in an extract of edible brown alga, Hijikia fusiforme (Hijiki). J Sci Food Agric 72(4):455–460
Peixoto MJ, Leitón ES, Pereira LF, Queiroz A, Magalhães F, Pereira R, Abreu H, Reis PA, Goncalves J.M, Ozório ROA (2016) Role of dietary seaweed supplementation on growth performance, digestive capacity and immune and stress responsiveness in European seabass (Dicentrarchus labrax). Aquaculture Rep 3: 189–197.
Ramberg JE, Nelson ED, Sinnott RA (2010) Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutr J 9:54. https://doi.org/10.1186/1475-2891-9-54
Refstie S, Korsøen ØJ, Storebakken T, Baeverfjord G, Lein I, Roem AJ (2000) Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture 190:49–63
Refstie S, Baeverfjord G, Seim RR, Elvebø O (2010) Effects of dietary yeast cell wall β-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture 305:109–116
Reilly P, O’Doherty JV, Pierce KM, Callan JJ, O'Sullivan JT, Sweeney T (2008) The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal 2(10):1465–1473
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MA (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol 25:114–123
Setyawan A, Isnansetyo A, Murwantoko IS, Handayani CR (2018) Comparative immune response of dietary fucoidan from three Indonesian brown algae in white shrimp Litopenaeus vannamei. AACL Bioflux 11(6):1707–1723
Shimei L, Yu P, Lin L, Li L (2011) Effects of dietary b-1,3-glucan, chitosan or raffinose on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Fish Shellfish Immunol 31:788–794
Shoemaker CA, Klesius PH, Lim C, Yildirim M (2003) Feed deprivation of channel catfish, Ictalurus punctatus (Rafinesque), influences organosomatic indices, chemical composition and susceptibility to Flavobacterium columnare. J Fish Dis 25:558–561
Sinurat E, Saepudin E, Peranginangin R, Hudiyono S (2016) Immuno-stimulatory activity of brown seaweed-derived fucoidans at different molecular weights and purity levels towards white spot syndrome virus (WSSV) in shrimp Litopenaeus vannamei. J Appl Pharm Sci 6(10):82–91
Sivagnanavelmurugan M, Thaddaeus BJ, Palavesam A, Immanuel G (2014) Dietary effect of Sargassum wightii fucoidan to enhance growth, prophenoloxidase gene expression of Penaeus monodon and immune resistance to Vibrio parahaemolyticus. Fish and Shellfish Immunology 39(2):439–449
Sony NM, Ishikawa M, Hossain MS, Koshio S, Yokoyama S (2019) The effect of dietary fucoidan on growth, immune functions, blood characteristics and oxidative stress resistance of juvenile red sea bream, Pagrus major. Fish Physiol Biochem 45(1):439–454
Sridee N, Boonanuntanasarn S (2012) The effects of food deprivation on hematological indices and blood indicators of liver function in Oxyleotris marmorata. World Acad Sci Eng Technol 6(5):248–252
Storebakken T, Refstie S, Ruyter B (2000) Soy products as fat and protein sources in fish feeds for intensive aquaculture. In: Drackley JK (ed) Soy in Animal Nutrition. Fed Anim. Sci. Sco, Savoy, IL, USA, pp 127–170
Takagi S, Shimeno S, Hosokawa H, Ukawa M (2001) Effect of lysine and methionine supplementation to a soy protein concentrate diet for red sea bream Pagrus major. Fish Sci 67:1088–1096
Takahashi Y, Uehara K, Watanabe R, Okumura T, Yamashita T, Omura H et al (1998) Efficacy of oral administration of fucoidan, a sulfated polysaccharide in controlling white spot syndrome in kuruma shrimp in Japan. In: Flegel TW (ed) Advance in shrimp biotechnology. Bangkok, National Center for Genetic Engineering and Biotechnology, pp 171–173
Tovar-Ram´ırez D, Mazurais D, Gatesoupe JF, Patrick Q, Chantal C, Zambonino-Infante JL (2010) Dietary probiotic live yeastmodulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture 300(1–4):142–147
Traifalgar RF, Kira H, Tung HT, Michael FR, Yokoyama ALS, Ishikaw MA, Koshio S (2010) Influence of dietary fucoidan supplementation on growth and immunological response of juvenile Marsupenaeus japonicus. J World Aquac Soc 41:235–244
Tuller J, Santis CD, Jerry DR (2014) Dietary influence of fucoidan supplementation on growth of Lates calcarifer (Bloch). Aquac Res 45:749–754
Van den Ingh T, Krogdahl Å, Olli JJ, Hendriks H, Koninkx J (1991) Effects of soybean-containing diets on the proximal and distal intestine in Atlantic salmon (Salmo salar): a morphological study. Aquaculture 94:297–305
Van den Ingh TSGAM, Olli JJ, Krogdahl Å (1996) Alcohol-soluble components in soybeans cause morphological changes in the distal intestine of Atlantic salmon, Salmo salar, L. J Fish Dis 19:47–53
Walsh AM, Sweeney T, O’Shea CJ, Doyle DN, O'Doherty JV (2013) Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br J Nutr 110:1630–1638
Wei WQ, Cong JB, Xian H, Zhang QJ, Hu YJ, Wu K et al (2001) The effect of polysaccharides from seaweed (SPS) on regulating the immune function in mice. Chin. J. New Drugs 10(9):35–37
Xing YH (2005) The regulation of FPS on blood lipids and its mechanism in hyperlipidemia animals. Guangzhou University of Chinese Medicine pp. 1-43.
Xu QY, Wang CA, Zhao ZG, Luo L (2012) Effects of replacement of fish meal by soy protein isolate on the growth, digestive enzyme activity and serum biochemical parameters for juvenile amur sturgeon (Acipenser schrenckii). Asian-Aust J Anim Sci 25(11):1588–1594
Yagi K (1998) Simple assay for the level of total lipid peroxides in serum or plasma. Methods in Molecular Biology 108:101–106
Yang Q, Yang R, Li M, Zhou Q, Liang X, Elmada ZC (2014) Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish and shellfish Immunol 41:264–270
Yone Y, Furuichi M, Urano K (1986) Effects of dietary wakame Undaria pinnatifida and Ascophyllum nodosum supplements on growth, feed efficiency, and proximate compositions of liver and muscle of red sea bream. Bull Japan Soc Sci Fish 52:1465–1488
Acknowledgments
Thanks are also due to Mr. Serge Dossou, Mr. Kokubu, and Mr. Taniguchi for their technical and logistic support during feeding trial and sample analysis.
Funding
This study was supported by the Management Expenses Grants of the United Graduate School of Agricultural Sciences, Kagoshima University, provided to Dr. Shunsuke Koshio.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sony, N.M., Hossain, M.S., Ishikawa, M. et al. Efficacy of mozuku fucoidan in alternative protein-based diet to improve growth, health performance, and stress resistance of juvenile red sea bream, Pagrus major. Fish Physiol Biochem 46, 2437–2455 (2020). https://doi.org/10.1007/s10695-020-00881-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00881-x