Abstract
This review covers nearly 20 years of studies on the ecology, physiology and genetics of the Hymenoptera Cotesia sesamiae, an African parasitoid of Lepidoptera that reduces populations of common maize borers in East and South Africa. The first part of the review presents studies based on sampling of C. sesamiae from maize crops in Kenya. From this agrosystem including one host plant and three main host borer species, studies revealed two genetically differentiated populations of C. sesamiae species adapted to their local host community, and showed that their differentiation involved the joint evolution of virulence genes and sensory mechanisms of host acceptance, reinforced by reproductive incompatibility due to Wolbachia infection status and natural inbreeding. In the second part, we consider the larger ecosystem of wild Poales plant species hosting many Lepidoptera stem borer species that are potential hosts for C. sesamiae. The hypothesis of other host-adapted C. sesamiae populations was investigated based on a large sampling of stem borer larvae on various Poales across sub-Saharan Africa. The sampling provided information on the respective contribution of local hosts, biogeography and Wolbachia in the genetic structure of C. sesamiae populations. Molecular evolution analyses highlighted that several bracovirus genes were under positive selection, some of them being under different selection pressure in C. sesamiae populations adapted to different hosts. This suggests that C. sesamiae host races result from co-evolution acting at the local scale on different bracovirus genes. The third part considers the mechanisms driving specialization. C. sesamiae host races are more or less host-specialized. This character is crucial for efficient and environmentally-safe use of natural enemies for biological control of pests. One method to get an insight in the evolutionary stability of host-parasite associations is to characterize the phylogenetic relationships between the so-called host-races. Based on the construction of a phylogeny of C. sesamiae samples from various host- and plant species, we revealed three main lineages. Mechanisms of differentiation are discussed with regard to the geography and ecology of the samples. One of the lineage presented all the hallmarks of a distinct species, which has been morphologically described and is now studied in the perspective of being used as biological control agent against Sesamia nonagrioides Lefèbvre (Lepidoptera: Noctuidae), a major maize pest in West Africa and Mediterranean countries (see Benoist et al. 2017). The fourth part reviews past and present use of C. sesamiae in biological control, and points out the interest of such molecular ecology studies to reconcile biodiversity and food security stakes in future biological control.



Credit: B. Le Ru

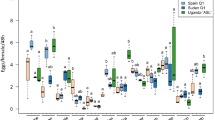
(adapted from Branca et al. 2011), with the associated host range, showing that allelic populations vary from polyphagy to monophagy


Similar content being viewed by others
Notes
Botanical order including 16 families among which Poaceae (ex Gramineae), Cyperaceae and Typhaceae are the main plant families host for stem borers parasitized by species of the C. flavipes complex.
References
Asgari S, Schmidt O (2002) A coiled-coil region of an insect immune suppressor protein is involved in binding and uptake by hemocytes. Insect Biochem Mol Biol 32:497–504
Asgari S, Hellers M, Schmidt O (1996) Host haemocyte inactivation by an insect parasitoid: transient expression of a polydnavirus gene. J Gen Virol 77:2653–2662. doi:10.1099/0022-1317-77-10-2653
Asgari S, Schmidt O, Theopold U (1997) A polydnavirus-encoded protein of an endoparasitoid wasp is an immune suppressor. J Gen Virol 78(Pt 11):3061–3070. doi:10.1099/0022-1317-78-11-3061
Baer CF, Tripp DW, Bjorksten TA, Antolin MF (2004) Phylogeography of a parasitoid wasp (Diaeretiella rapae): no evidence of host-associated lineages. Mol Ecol 13:1859–1869. doi:10.1111/j.1365-294X.2004.02196.x
Bale J, van Lenteren J, Bigler F (2008) Biological control and sustainable food production. Philos Trans R Soc B 363:761–776. doi:10.1098/rstb.2007.2182
Benoist R, Chantre C, Capdevielle‑Dulac C, Bodet M, Mougel F, Calatayud PA, Dupas S, Huguet E, Jeannette R, Obonyo J, Odorico C, Silvain JF, Le Ru B, Kaiser L (2017) Relationship between oviposition, virulence gene expression and parasitism success in Cotesia typhae nov. sp. parasitoid strains. Genetica. doi:10.1007/s10709-017-9987-5 (this issue)
Bézier A, Annaheim M, Herbinière J, Wetterwald C, Gyapay G, Bernard-Samain S, Wincker P, Roditi I, Heller M, Belghazi M, Pfister-Wilhem R, Periquet G, Dupuy C, Huguet E, Volkoff A-N, Lanzrein B, Drezen J-M (2009) Polydnaviruses of braconid wasps derive from an ancestral nudivirus. Science 323:926–930. doi:10.1126/science.1166788
Bézier A, Louis F, Jancek S, Periquet G, Theze J, Gyapay G, Musset K, Lesobre J, Lenoble P, Dupuy C, Gundersen-Rindal D, Herniou EA, Drezen J-M (2013) Functional endogenous viral elements in the genome of the parasitoid wasp Cotesia congregata: insights into the evolutionary dynamics of bracoviruses. Philos Trans R Soc B 368:20130047–20130047. doi:10.1098/rstb.2013.0047
Branca A, Le Ru BP, Vavre F, Silvain J-F, Dupas S (2011) Intraspecific specialization of the generalist parasitoid Cotesia sesamiae revealed by polydnavirus polymorphism and associated with different Wolbachia infection. Mol Ecol 20:959–971. doi:10.1111/j.1365-294X.2010.04977.x
Brodeur J (2012) Host specificity in biological control: insights from opportunistic pathogens. Evol Appl 5:470–480. doi:10.1111/j.1752-4571.2012.00273.x
Chabi-Olaye A, Nolte C, Schulthess F, Borgemeister C (2006) Role of inland valleys in the management of stem borers and their natural enemies in upland maize fields in the humid forest zone of Cameroon. Environ Entomol 35:282–292
Chevignon G, Thézé J, Cambier S, Poulain J, Silva CD, Bezier A, Musset K, Moreau SJM, Drezen J-M, Huguet E (2014) Functional annotation of Cotesia congregata bracovirus: identification of the viral genes expressed in parasitized host immune tissues. J Virol. doi:10.1128/JVI.00209-14
Danley PD, Husemann M, Ding B, DiPietro LM, Beverly EJ, Peppe DJ (2012) The impact of the geologic history and paleoclimate on the diversification of East african cichlids. Int J Evol Biol 2012:1–20. doi:10.1155/2012/574851
Davis J, Stamps J (2004) The effect of natal experience on habitat preferences. Trends Ecol Evol 19:411–416. doi:10.1016/j.tree.2004.04.006
Dinardo-Miranda LL, Fracasso JV, da Costa VP, Lopes DOT (2014) Dispersal of Cotesia flavipes in sugarcane field and implications for parasitoid releases. Bragantia 73:163–170. doi:10.1590/brag.2014.023
Dupas S, Gitau C, Le Rü B, Silvain J-F (2006) Single-step PCR differentiation of Cotesia sesamiae (Cameron) and Cotesia flavipes Cameron (Hymenoptera: Braconidae) using polydnavirus markers. Ann Soc Entomol Fr 42:319–323
Dupas S, Gitau CW, Branca A, Le Rü BP, Silvain J-F (2008) Evolution of a polydnavirus gene in relation to parasitoid-host species immune resistance. J Hered 99:491–499. doi:10.1093/jhered/esn047
Faria R, Renaut S, Galindo J, Pinho C, Melo-Ferreira J, Melo M, Jones F, Salzburger W, Schluter D, Butlin R (2014) Advances in ecological speciation: an integrative approach. Mol Ecol 23:513–521. doi:10.1111/mec.12616
Gauthier J (2017) Génomique de l'adaptation des guêpes parasitoïdes du genre Cotesia: rôle du bracovirus. PhD thesis, University François Rabelais, Tours, 267p
Gauthier J, Drezen J-M, Herniou EA (2017) The recurrent domestication of viruses: major evolutionary transitions in parasitic wasps. Parasitol. doi:10.1017/S0031182017000725
Gitau CW, Gundersen-Rindal D, Pedroni M, Mbugi PJ, Dupas S (2007) Differential expression of the CrV1 haemocyte inactivation-associated polydnavirus gene in the African maize stem borer Busseola fusca (Fuller) parasitized by two biotypes of the endoparasitoid Cotesia sesamiae (Cameron). J Insect Physiol 53:676–684. doi:10.1016/j.jinsphys.2007.04.008
Gitau CW, Schulthess F, Stephane D (2010) An association between host acceptance and virulence status of different populations of Cotesia sesamiae, a braconid larval parasitoid of lepidopteran cereal stemborers in Kenya. Biol Control 54:100–106. doi:10.1016/j.biocontrol.2010.04.010
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, Princeton
Gounou S, Jiang N, Schulthess F (2009) Long-term seasonal fluctuations of lepidopteran cereal stemborers and their natural enemies on maize and wild host plants in southern Benin. Insect Sci 16:329–341. doi:10.1111/j.1744-7917.2009.01264.x
Hawkins BA (1994) Pattern and process in host-parasitoid interactions. Cambridge University Press, Cambridge
Herniou EA, Huguet E, Theze J, Bezier A, Periquet G, Drezen J-M (2013) When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses. Philos Trans R Soc B. 368:20130051. doi:10.1098/rstb.2013.0051
Jancek S, Bézier A, Gayral P, Paillusson C, Kaiser L, Dupas S, Le Ru BP, Barbe V, Periquet G, Drezen J-M, Herniou EA (2013) Adaptive selection on bracovirus genomes drives the specialization of Cotesia parasitoid wasps. PLoS ONE 8:e64432. doi:10.1371/journal.pone.0064432
Kaiser L, Couty A, Perez-Maluf R (2009) Dynamic use of fruit odours to locate host larvae: individual learning, physiological state and genetic variability as adaptive mechanisms. Adv Parasitol 70: 67–95. doi:10.1016/S0065-308X(09)70003-X
Kaiser L, Le Ru BP, Kaoula F, Paillusson C, Capdevielle-Dulac C, Obonyo JO, Herniou EA, Jancek S, Branca A, Calatayud P-A, Silvain J-F, Dupas S (2015) Ongoing ecological speciation in Cotesia sesamiae, a biological control agent of cereal stem borers. Evol Appl 8:807–820. doi:10.1111/eva.12260
Kaiser L, Fernandez-Triana J, Capdevielle-Dulac C, Chantre C, Bodet M, Kaoula F, Benoist R, Calatayud P-A, Dupas S, Herniou EA, Jeannette R, Obonyo J, Silvain J-F, Le Ru B (2017) Systematics and biology of Cotesia typhae sp. n. (Hymenoptera, Braconidae, Microgastrinae), a potential biological control agent against the noctuid Mediterranean corn borer, Sesamia nonagrioides. ZooKeys 682:105–136. doi:10.3897/zookeys.682.13016
Kankonda OM, Akaibe BD, Sylvain NM, Le Ru B-P (2017) Response of maize stemborers and associated parasitoids to the spread of grasses in the rainforest zone of Kisangani, DR Congo: effect on stemborers biological control: response of stemborers and parasitoids to grasses spreading. Agric For Entomol. doi:10.1111/afe.12238
Kergoat GJ, Toussaint EFA, Capdevielle-Dulac C, Clamens A-L, Ong’amo G, Conlong D, van Den Berg J, Cugala D, Pallangyo B, Mubenga O, Chipabika G, Ndemah R, Sezonlin M, Bani G, Molo R, Ali A, Calatayud P-A, Kaiser L, Silvain J-F, Le Ru B (2015) Integrative taxonomy reveals six new species related to the Mediterranean corn stalk borer Sesamia nonagrioides (Lefèbvre) (Lepidoptera, Noctuidae, Sesamiina). Zool J Linn Soc 175:244–270. doi:10.1111/zoj.12275
Kester KM, Barbosa P (1991) Postemergence learning in the insect parasitoid, Cotesia congregata (Say) (Hymenoptera: Braconidae). J Insect Behav 4:727–742. doi:10.1007/BF01052227
Kfir R (1995) Parasitoids of the African stem borer, Busseola fusca (Lepidoptera: Noctuidae), in South Africa. Bull Entomol Res 85:369. doi:10.1017/S0007485300036105
Kfir R, Bell RA (1993) Intraseasonal changes in populations of the African stem borer Busseola fusca (Fuller) (Lepidoptera: Noctuidae) and its parasitoids in Natal, South Africa. Rev Zool Afr 107:543–553
Kfir R, Overholt WA, Khan ZR, Polaszek A (2002) Biology and management of economically important lepidopteran cereal stem borers in Africa. Annu Rev Entomol 47:701–731. doi:10.1146/annurev.ento.47.091201.145254
Le Rü BP, Ong’amo GO, Moyal P, Muchugu E, Ngala L, Musyoka B, Abdullah Z, Matama-Kauma T, Lada VY, Pallangyo B, Omwega CO, Schulthess F, Calatayud P-A, Silvain J-F (2006) Geographic distribution and host plant ranges of East African noctuid stem borers. Ann Soc Entomol Fr NS 42:353–361. doi:10.1080/00379271.2006.10697467
Mailafiya DM, Ru BPL, Kairu EW, Calatayud P-A, Dupas S (2010) Geographic distribution, host range and perennation of Cotesia sesamiae and Cotesia flavipes Cameron in cultivated and natural habitats in Kenya. Biol Control 54:1–8. doi:10.1016/j.biocontrol.2009.11.008
Midingoyi SG, Affognon HD, Macharia I, Ong’amo G, Abonyo E, Ogola G, Groote HD, LeRu B (2016) Assessing the long-term welfare effects of the biological control of cereal stemborer pests in East and Southern Africa: evidence from Kenya, Mozambique and Zambia. Agric Ecosyst Environ 230:10–23. doi:10.1016/j.agee.2016.05.026
Mochiah MB, Ngi-Song AJ, Overholt WA, Botchey M (2001) Host Suitability of Four Cereal Stem Borers (Lepidoptera: Crambidae, Noctuidae) for Different Geographic Populations of Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae) in Kenya. Biol Control 21:285–292. doi:10.1006/bcon.2001.0941
Mochiah MB, Ngi-Song AJ, Overholt WA, Stouthamer R (2002) Wolbachia infection in Cotesia sesamiae (Hymenoptera: Braconidae) causes cytoplasmic incompatibility: implications for biological control. Biol Control 25:74–80
Mohyuddin A, Greathead D (1970) An annotated list of parasites of graminaceous stem borers in East Africa, with a discussion on their potential in biological control. Entomophaga 15:241–274
Moolman J, Van den Berg J, Conlong D, Cugala D, Siebert S, Le Ru B (2014) Species diversity and distribution of lepidopteran stem borers in South Africa and Mozambique. J Appl Entomol 138:52–66. doi:10.1111/jen.12085
Muirhead K, Austin A, Sallam M (2008) The systematics and biology of Cotesia nonagriae (Olliff) stat. rev.(Hymenoptera: Braconidae: Microgastrinae), a newly recognized member of the Cotesia flavipes species complex. Zootaxa 1846:35–46
Muirhead KA, Murphy NP, Sallam N, Donnellan SC, Austin AD (2012) Phylogenetics and genetic diversity of the Cotesia flavipes complex of parasitoid wasps (Hymenoptera: Braconidae), biological control agents of lepidopteran stemborers. Mol Phylogenet Evol 63:904–914. doi:10.1016/j.ympev.2012.03.003
Ndemah R, Schulthess F (2002) Yield of maize in relation to natural field infestations and damage by Lepidopteran borers in the forest and forest/savanna transition zones of Cameroon. Int J Trop Insect Sci 22:183–192. doi:10.1017/S1742758400012030
Ndemah R, Schulthess F, Abang A, Ghogomu RT, Ntonifor N, Dupas S, LeRü B (2012) Suitability of cereal stemborers in Cameroon to Kenyan populations of the braconid larval parasitoid Cotesia sesamiae: suitability of cereal stemborers. J Appl Entomol 136:60–69. doi:10.1111/j.1439-0418.2010.01585.x
Ngi-Song AJ, Overholt WA, Ayertey JN (1995) Suitability of African gramineous stemborers for development of. Cotesia flavipes and C. sesamiae (Hymenoptera: Braconidae). Environ Entomol. 24:978–984
Ngi-Song AJ, Overholt WA, Stouthamer R (1998) Suitability of Busseola fusca and Sesamia calamistis (Lepidoptera: Noctuidae) for the development of two populations of Cotesia sesamiae (Hymenoptera: Braconidae) in Kenya. Biol Control 12:208–214
Ong’amo GO, Rü BPL, Dupas S, Moyal P, Muchugu E, Calatayud P-A, Silvain J-F (2006) The role of wild host plants in the abundance of lepidopteran stem borers along altitudinal gradient in Kenya. Ann Société Entomol Fr NS 42:363–370. doi:10.1080/00379271.2006.10697468
Ong’amo GO, Gall PL, Ndemah R, Ru BPL (2014) Diversity and host range of lepidopteran stemborer species in Cameroon. Afr Entomol 22:625–635. doi:10.4001/003.022.0316
Ong’amo GO, Pallangyo B, Ali A, Njaku M, Musyoka B, Le Ru BP (2018) Diversity and abundance of lepidopteran stem borers and their respective native hosts in different vegetation mosaics in Tanzania. African Entomologist (accepted)
Otieno NA, Rü BPL, Ong’amo GO, Dupas S, Calatayud P-A, Makobe M, Ochora J, Silvain J-F (2006) Diversity and abundance of wild host plants of lepidopteran stem borers in two different agroecological zones of Kenya. Ann Société Entomol Fr NS 42:371–380. doi:10.1080/00379271.2006.10697469
Overholt WA (2000) La lutte biologique. In: Polaszek A, Blary D, Delvare G (eds) Les foreurs des tiges de céréales en Afrique: importance économique, systématique, ennemis naturels et méthodes de lutte. Editions Quae. Cirad, Versailles, pp 351–366
Parisot C-J (2016) Les nouveaux guerriers des champs. Arte. http://boutique.arte.tv/f11358-nouveaux_guerriers_champs. Accessed 21 Sept 2017
Phillips CB, Vink CJ, Blanchet A, Hoelmer KA (2008) Hosts are more important than destinations: what genetic variation in Microctonus aethiopoides (Hymenoptera: Braconidae) means for foreign exploration for natural enemies. Mol Phylogenet Evol 49:467–476. doi:10.1016/j.ympev.2008.08.005
Polaszek A, Delvare G (2000) Les foreurs des tiges de céréales en Afrique: Importance économique, systématique, ennemis naturels et méthodes de lutte. Cirad, Versailles
Potting RPJ, Overholt WA, Danso FO, Takasu K (1997) Foraging behavior and life history of the stemborer parasitoid Cotesia flavipes (hymenoptera: Braconidae). J Insect Behav 10:13
Quicke DL (1997) Parasitic wasps. Springer, The Netherlands
Quicke DLJ (2014) The Braconid and ichneumonid parasitoid wasps: biology, systematics, evolution and ecology. Wiley, Chichester. doi:10.1002/9781118907085
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352. doi:10.1111/j.1461-0248.2004.00715.x
Sezonlin M, Dupas S, Le Rü B, Le Gall P, Moyal P, Calatayud P-A, Giffard I, Faure N, Silvain J-F (2006) Phylogeography and population genetics of the maize stalk borer Busseola fusca (Lepidoptera, Noctuidae) in sub-Saharan Africa. Mol Ecol 15:407–420. doi:10.1111/j.1365-294X.2005.02761.x
Smith MA, Rodriguez JJ, Whitfield JB, Deans AR, Janzen DH, Hallwachs W, Hebert PD (2008) Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc Natl Acad Sci USA 105:12359–12364
Stoltz DB, Xu D (1990) Polymorphism in polydnavirus genomes. Can J Microbiol 36:538–543. doi:10.1139/m90-094
Via S, Hawthorne DJ (2002) The genetic architecture of ecological specialization: correlated gene effects on host use and habitat choice in pea aphids. Am Nat 159:S76–S88. doi:10.1086/338374
Whitfield JB (1994) Mutualistic viruses and the evolution of host ranges in endoparasitoid Hymenoptera. In Hawkins BA, Sheehan W (eds) Parasitoid Community ecology. Oxford Science Publications. Oxford University Press, Oxford, pp 163–176
Whitfield JB (2000) Phylogeny of microgastroid braconid wasps, and what it tells us about polydnavirus evolution. In: Hymenoptera: evolution, biodiversity and biological control, Canberra, pp 97–105
Acknowledgements
The authors thank Geneviève Prevost for inviting them to present this work in this Thematic issue, and Rose Ndemah for her info on the use of Cotesia sesamiae as biological control agent in west Africa. This review project was supported by the ANR Bioadapt (ABC Papogen project ANR-12-ADAP-24 0001), and by the authors’ operating grants from IRD, CNRS, and ICIPE. R. Benoist is funded by the « école doctorale 227 MNHN-UPMC Sciences de la Nature et de l’Homme: évolution et écologie».
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaiser, L., Dupas, S., Branca, A. et al. The Cotesia sesamiae story: insight into host-range evolution in a Hymenoptera parasitoid and implication for its use in biological control programs. Genetica 145, 455–468 (2017). https://doi.org/10.1007/s10709-017-9989-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-017-9989-3