Abstract
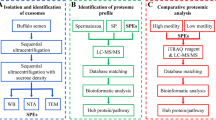
The surface of the spermatozoa is coated with glycoproteins the redistribution of which during in vitro capacitation plays a key role in the subsequent fertilization process. Lipid rafts are membrane microdomains involved in signal transduction through receptors and include or recruit specific types of proteins and glycoproteins. Few studies have focused on identifying glycoproteins resident in the lipid rafts of spermatozoa. Proteins associated with lipid rafts modify their localization during capacitation. The objective of the study was to identify the glycoproteins associated with lipid rafts of capacitated boar spermatozoa through a lectin-binding assay coupled to mass spectrometry approach. From the proteomic profiles generated by the raft proteins extractions, we observed that after capacitation the intensity of some bands increased while that of others decreased. To determine whether the proteins obtained from lipid rafts are glycosylated, lectin blot assays were performed. Protein bands with a good resolution and showing significant glycosylation modifications after capacitation were analyzed by mass spectrometry. The bands of interest had an apparent molecular weight of 64, 45, 36, 34, 24, 18 and 15 kDa. We sequenced the 7 bands and 20 known or potential glycoproteins were identified. According to us, for ten of them this is the first time that their association with sperm lipid rafts is described (ADAM5, SPMI, SPACA1, Seminal plasma protein pB1, PSP-I, MFGE8, tACE, PGK2, SUCLA2, MDH1). Moreover, LYDP4, SPAM-1, HSP60, ZPBP1, AK1 were previously reported in lipid rafts of mouse and human spermatozoa but not in boar spermatozoa. We also found and confirmed the presence of ACR, ACRBP, AWN, AQN3 and PRDX5 in lipid rafts of boar spermatozoa. This paper provides an overview of the glycosylation pattern in lipid rafts of boar spermatozoa before and after capacitation. Further glycomic analysis is needed to determine the type and the variation of glycan chains of the lipid rafts glycoproteins on the surface of spermatozoa during capacitation and acrosome reaction.



Similar content being viewed by others
References
Yanagimachi, R.: Mammalian fertilization. In: Knobil, E., Neill, J.D. (eds.) The Physiology of Reproduction, vol. I, pp. 189–317. Raven Press, New York (1994)
Flesch, F.M., Gadella, B.M.: Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta. 1469(3), 197–235 (2000)
Boerke, A., Tsai, P.S., Garcia-Gil, N., Brewis, I.A., Gadella, B.M.: Capacitation-dependent reorganization of microdomains in the apical sperm head plasma membrane: functional relationship with zona binding and the zona-induced acrosome reaction. Theriogenology. 70(8), 1188–1196 (2008)
Fierro, R., Foliguet, B., Grignon, G., Daniel, M., Bene, M.C., Faure, G.C., Barbarino-Monnier, P.: Lectin-binding sites on human sperm during acrosome reaction: modifications judged by electron microscopy/flow cytometry. Arch. Androl. 36(3), 187–196 (1996)
Jiménez, I., González-Márquez, H., Ortiz, R., Herrera, J.A., García, A., Betancourt, M., Fierro, R.: Changes in the distribution of lectin receptors during capacitation and acrosome reaction in boar spermatozoa. Theriogenology. 59, 1171–1180 (2003)
Talevi, R., Gualtieri, R.: Molecules involved in sperm-oviduct adhesion and release. Theriogenology. 73(6), 796–801 (2010doi:S0093-691X(09)00288-X [pii]). https://doi.org/10.1016/j.theriogenology.2009.07.005
Fierro, R., Bonilla, E., Casas, E., Jimenez, I., Ducolomb, Y., Betancourt, M.: Inhibition of pig oocyte in vitro fertilization by the action of components of the zona pellucida. Theriogenology. 42(2), 227–234 (1994) doi:0093-691X(94)90266-6 [pii]
Jiménez, I., González-Márquez, H., Ortiz, R., Betancourt, M., Herrera, J., Fierro, R.: Expression of lectin receptors en the membrane surface of sperm of fertile and subfertile boar by flow cytometry. Arch. Androl. 48, 159–166 (2002)
Ohta, K., Sato, C., Matsuda, T., Toriyama, M., Lennarz, W.J., Kitajima, K.: Isolation and characterization of low density detergent-insoluble membrane (LD-DIM) fraction from sea urchin sperm. Biochem. Biophys. Res. Commun. 258(3), 616–623 (1999)
Xu, H., Liu, F., Srakaew, N., Koppisetty, C., Nyholm, P.G., Carmona, E., Tanphaichitr, N.: Sperm arylsulfatase a binds to mZP2 and mZP3 glycoproteins in a nonenzymatic manner. Reproduction. 144(2), 209–219 (2012). https://doi.org/10.1530/REP-11-0338
Guimarães, D.B., Barros, T.B., van Tilburg, M.F., Martins, J.A.M., Moura, A.A., Moreno, F.B., Monteiro-Moreira, A.C., Moreira, R.A., Toniolli, R.: Sperm membrane proteins associated with the boar semen cryopreservation. Anim. Reprod. Sci. 183, 27–38 (2017) https://doi.org/10.1016/j.anireprosci.2017.06.005
Jiménez, I., Fierro, R., González-Márquez, H., Mendoza-Hernández, G., Romo, S.: M, B.: carbohydrate affinity chromatography indicates that arylsulfatase-a from capacitated boar sperm has mannose and N-acetylglucosamine/sialic acid residues. Arch. Androl. 52(6), 455–462 (2006)
Brown, D.A.: Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda). 21, 430–439 (2006). https://doi.org/10.1152/physiol.00032.2006
Fan, J., Sammalkorpi, M., Haataja, M.: Formation and regulation of lipid microdomains in cell membranes: theory, modeling, and speculation. FEBS Lett. 584(9), 1678–1684 (2010). https://doi.org/10.1016/j.febslet.2009.10.051
Helms, J.B., Zurzolo, C.: Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 5(4), 247–254 (2004)
Lingwood, D., Simons, K.: Lipid rafts as a membrane-organizing principle. Science. 327(5961), 46–50 (2010). https://doi.org/10.1126/science.1174621
Nyholm, T.K.: Lipid-protein interplay and lateral organization in biomembranes. Chem. Phys. Lipids. 189, 48–55 (2015). https://doi.org/10.1016/j.chemphyslip.2015.05.008
van Gestel, R.A., Brouwers, J.F., Ultee, A., Helms, J.B., Gadella, B.M.: Ultrastructure and lipid composition of detergent-resistant membranes derived from mammalian sperm and two types of epithelial cells. Cell Tissue Res. 363(1), 129–145 (2016). https://doi.org/10.1007/s00441-015-2272-y
Pike, L.J.: Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J. Lipid Res. 47(7), 1597–1598 (2006). https://doi.org/10.1194/jlr.E600002-JLR200
Alonso, M.A., Millan, J.: The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J. Cell Sci. 114(Pt 22), 3957–3965 (2001)
Brown, D.A., London, E.: Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275(23), 17221–17224 (2000). https://doi.org/10.1074/jbc.R000005200
Brown, D.A., Rose, J.K.: Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68(3), 533–544 (1992) doi:0092-8674(92)90189-J [pii]
Cross, N.L.: Reorganization of lipid rafts during capacitation of human sperm. Biol. Reprod. 71(4), 1367–1373 (2004)
Shadan, S., James, P.S., Howes, E.A., Jones, R.: Cholesterol efflux alters lipid raft stability and distribution during capacitation of boar spermatozoa. Biol. Reprod. 71(1), 253–265 (2004)
Sleight, S.B., Miranda, P.V., Plaskett, N.W., Maier, B., Lysiak, J., Scrable, H., Herr, J.C., Visconti, P.E.: Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol. Reprod. 73(4), 721–729 (2005)
Travis, A.J., Merdiushev, T., Vargas, L.A., Jones, B.H., Purdon, M.A., Nipper, R.W., Galatioto, J., Moss, S.B., Hunnicutt, G.R., Kopf, G.S.: Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and Guinea pig spermatozoa. Dev. Biol. 240(2), 599–610 (2001)
Miranda, P.V., Allaire, A., Sosnik, J., Visconti, P.E.: Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biol. Reprod. 80(5), 897–904 (2009)
Kasekarn, W., Kanazawa, T., Hori, K., Tsuchiyama, T., Lian, X., Garenaux, E., Kongmanas, K., Tanphaichitr, N., Yasue, H., Sato, C., Kitajima, K.: Pig sperm membrane microdomains contain a highly glycosylated 15-25-kDa wheat germ agglutinin-binding protein. Biochem. Biophys. Res. Commun. 426(3), 356–362 (2012). https://doi.org/10.1016/j.bbrc.2012.08.090
de Laurentiis, A., Donovan, L., Arcaro, A.: Lipid rafts and caveolae in signaling by growth factor receptors. Open Biochemistry Journal. 1, 12–32 (2007)
Botto, L., Bernabo, N., Palestini, P., Barboni, B.: Bicarbonate induces membrane reorganization and CBR1 and TRPV1 endocannabinoid receptor migration in lipid microdomains in capacitating boar spermatozoa. J. Membr. Biol. 238(1–3), 33–41 (2010). https://doi.org/10.1007/s00232-010-9316-8
Nixon, B., Mitchell, L.A., Anderson, A.L., McLaughlin, E.A., O'Bryan, M.K., Aitken, R.J.: Proteomic and functional analysis of human sperm detergent resistant membranes. J. Cell. Physiol. 226(10), 2651–2665 (2011). https://doi.org/10.1002/jcp.22615
Peterson, G.L.: A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83(2), 346–356 (1977)
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680–685 (1970)
Vercoutter-Edouart, A.S., Slomianny, M.C., Dekeyzer-Beseme, O., Haeuw, J.F., Michalski, J.C.: Glycoproteomics and glycomics investigation of membrane N-glycosylproteins from human colon carcinoma cells. Proteomics. 8(16), 3236–3256 (2008). https://doi.org/10.1002/pmic.200800151
Schneider, C.A., Rasband, W.S., Eliceiri, K.W.: NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 9(7), 671–675 (2012)
Romero-Calvo, I., Ocon, B., Martinez-Moya, P., Suarez, M.D., Zarzuelo, A., Martinez-Augustin, O., de Medina, F.S.: Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 401(2), 318–320 (2010). https://doi.org/10.1016/j.ab.2010.02.036
Gurcel, C., Vercoutter-Edouart, A.S., Fonbonne, C., Mortuaire, M., Salvador, A., Michalski, J.C., Lemoine, J.: Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal. Bioanal. Chem. 390(8), 2089–2097 (2008). https://doi.org/10.1007/s00216-008-1950-y
Boerke, A., van der Lit, J., Lolicato, F., Stout, T.A., Helms, J.B., Gadella, B.M.: Removal of GPI-anchored membrane proteins causes clustering of lipid microdomains in the apical head area of porcine sperm. Theriogenology. 81(4), 613–624 (2014). https://doi.org/10.1016/j.theriogenology.2013.11.014
van Gestel, R.A., Brewis, I.A., Ashton, P.R., Helms, J.B., Brouwers, J.F., Gadella, B.M.: Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells. Mol. Hum. Reprod. 11(8), 583–590 (2005)
Selvaraj, V., Asano, A., Buttke, D.E., McElwee, J.L., Nelson, J.L., Wolff, C.A., Merdiushev, T., Fornes, M.W., Cohen, A.W., Lisanti, M.P., Rothblat, G.H., Kopf, G.S., Travis, A.J.: Segregation of micron-scale membrane sub-domains in live murine sperm. J. Cell. Physiol. 206(3), 636–646 (2006)
Selvaraj, V., Buttke, D.E., Asano, A., McElwee, J.L., Wolff, C.A., Nelson, J.L., Klaus, A.V., Hunnicutt, G.R., Travis, A.J.: GM1 dynamics as a marker for membrane changes associated with the process of capacitation in murine and bovine spermatozoa. J. Androl. 28(4), 588–599 (2007). https://doi.org/10.2164/jandrol.106.002279
Nixon, B., Aitken, R.J.: The biological significance of detergent-resistant membranes in spermatozoa. J. Reprod. Immunol. 83(1–2), 8–13 (2009)
Asano, A., Selvaraj, V., Buttke, D.E., Nelson, J.L., Green, K.M., Evans, J.E., Travis, A.J.: Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts. J. Cell. Physiol. 218(3), 537–548 (2009)
Garofalo, T., Manganelli, V., Grasso, M., Mattei, V., Ferri, A., Misasi, R., Sorice, M.: Role of mitochondrial raft-like microdomains in the regulation of cell apoptosis. Apoptosis. 20(5), 621–634 (2015). https://doi.org/10.1007/s10495-015-1100-x
Watanabe, H., Kondoh, G.: Mouse sperm undergo GPI-anchored protein release associated with lipid raft reorganization and acrosome reaction to acquire fertility. J. Cell Sci. 124(Pt 15), 2573–2581 (2011). https://doi.org/10.1242/jcs.086967
Asquith, K.L., Baleato, R.M., McLaughlin, E.A., Nixon, B., Aitken, R.J.: Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J. Cell Sci. 117(Pt 16), 3645–3657 (2004). https://doi.org/10.1242/jcs.01214
Kongmanas, K., Kruevaisayawan, H., Saewu, A., Sugeng, C., Fernandes, J., Souda, P., Angel, J.B., Faull, K.F., Aitken, R.J., Whitelegge, J., Hardy, D., Berger, T., Baker, M.A., Tanphaichitr, N.: Proteomic characterization of pig sperm anterior head plasma membrane reveals roles of Acrosomal proteins in ZP3 binding. J. Cell. Physiol. 230(2), 449–463 (2015). https://doi.org/10.1002/jcp.24728
Marchand, S., Devillers-Thiery, A., Pons, S., Changeux, J.P., Cartaud, J.: Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J. Neurosci. 22(20), 8891–8901 (2002)
Minogue, S., Waugh, M.G.: Lipid rafts, microdomain heterogeneity and inter-organelle contacts: impacts on membrane preparation for proteomic studies. Biol. Cell. 104(10), 618–627 (2012). https://doi.org/10.1111/boc.201200020
Wassarman, P.M., Litscher, E.S.: Towards the molecular basis of sperm and egg interaction during mammalian fertilization. Cells Tissues Organs. 168(1–2), 36–45 (2001)
Ducolomb, Y., Gonzalez-Marquez, H., Fierro, R., Jimenez, I., Casas, E., Flores, D., Bonilla, E., Salazar, Z., Betancourt, M.: Effect of porcine follicular fluid proteins and peptides on oocyte maturation and their subsequent effect on in vitro fertilization. Theriogenology. 79(6), 896–904 (2013). https://doi.org/10.1016/j.theriogenology.2013.01.024
Day, A.E., Quilter, C.R., Sargent, C.A., Mileham, A.J.: Characterization of the porcine sperm adhesion molecule gene SPAM1- expression analysis, genomic structure, and chromosomal mapping. Anim. Genet. 33(3), 211–214 (2002)
Green, C.E., Bredl, J., Holt, W.V., Watson, P.F., Fazeli, A.: Carbohydrate mediation of boar sperm binding to oviductal epithelial cells in vitro. Reproduction. 122(2), 305–315 (2001)
Amari, S., Yonezawa, N., Mitsui, S., Katsumata, T., Hamano, S., Kuwayama, M., Hashimoto, Y., Suzuki, A., Takeda, Y., Nakano, M.: Essential role of the nonreducing terminal alpha-mannosyl residues of the N-linked carbohydrate chain of bovine zona pellucida glycoproteins in sperm-egg binding. Mol. Reprod. Dev. 59(2), 221–226 (2001). https://doi.org/10.1002/mrd.1026
Nixon, B., Lu, Q., Wassler, M.J., Foote, C.I., Ensslin, M.A., Shur, B.D.: Galactosyltransferase function during mammalian fertilization. Cells Tissues Organs. 168(1–2), 46–57 (2001)
Esteves, S.C., Sharma, R.K., Thomas Jr., A.J., Agarwal, A.: Evaluation of acrosomal status and sperm viability in fresh and cryopreserved specimens by the use of fluorescent peanut agglutinin lectin in conjunction with hypo-osmotic swelling test. Int. Braz. J. Urol. 33(3), 364–374 (2007)
Aleksinskaya, M.A., Nikolaeva, M.A., Danilov, S.M., Elistratova, O.S., Sukhikh, G.T.: Quantitative study of testicular angiotensin-converting enzyme on the surface of human spermatozoa. Bull. Exp. Biol. Med. 141(1), 36–39 (2006)
Hagaman, J.R., Moyer, J.S., Bachman, E.S., Sibony, M., Magyar, P.L., Welch, J.E., Smithies, O., Krege, J.H., O'Brien, D.A.: Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. 95(5), 2552–2557 (1998)
Yu, X.C., Sturrock, E.D., Wu, Z., Biemann, K., Ehlers, M.R., Riordan, J.F.: Identification of N-linked glycosylation sites in human testis angiotensin-converting enzyme and expression of an active deglycosylated form. J. Biol. Chem. 272(6), 3511–3519 (1997)
Lassalle, B., Testart, J.: Human zona pellucida recognition associated with removal of sialic acid from human sperm surface. J. Reprod. Fertil. 101(3), 703–711 (1994)
Froman, D.P., Engel Jr., H.N.: Alteration of the spermatozoal glycocalyx and its effect on duration of fertility in the fowl (Gallus domesticus). Biol. Reprod. 40(3), 615–621 (1989)
Topfer-Petersen, E., Romero, A., Varela, P.F., Ekhlasi-Hundrieser, M., Dostalova, Z., Sanz, L., Calvete, J.J.: Spermadhesins: a new protein family. Facts, hypotheses and perspectives. Andrologia. 30(4–5), 217–224 (1998)
Ekhlasi-Hundrieser, M., Gohr, K., Wagner, A., Tsolova, M., Petrunkina, A., Topfer-Petersen, E.: Spermadhesin AQN1 is a candidate receptor molecule involved in the formation of the oviductal sperm reservoir in the pig. Biol. Reprod. 73(3), 536–545 (2005). https://doi.org/10.1095/biolreprod.105.040824
Rodriguez-Martinez, H., Saravia, F., Wallgren, M., Martinez, E.A., Sanz, L., Roca, J., Vazquez, J.M., Calvete, J.J.: Spermadhesin PSP-I/PSP-II heterodimer induces migration of polymorphonuclear neutrophils into the uterine cavity of the sow. J. Reprod. Immunol. 84(1), 57–65 (2010). https://doi.org/10.1016/j.jri.2009.10.007
Nixon, B., Asquith, K.L., John Aitken, R.: The role of molecular chaperones in mouse sperm-egg interactions. Mol. Cell. Endocrinol. 240(1–2), 1–10 (2005). https://doi.org/10.1016/j.mce.2005.06.004
Reid, A.T., Redgrove, K., Aitken, R.J., Nixon, B.: Cellular mechanisms regulating sperm-zona pellucida interaction. Asian Journal of Andrology. 13(1), 88–96 (2011). https://doi.org/10.1038/aja.2010.74
List of abbreviations
AR: acrosome reaction; C: capacitated; DIG: digoxigenin; DRM: detergent-resistant membrane; DSA: Datura stramonium agglutinin; Gal: galactose; GlcNAc: N-acetylglucosamine; GM1: ganglioside M1; GNA: Galanthus nivalis agglutinin; Man: mannose; NC: non-capacitated; NeuAc: sialic acid; OD: optical density; PNA: peanut agglutinin; RP: raft proteins; RPC: raft protein of C; RPNC: raft protein of NC; SNA: Sambucus nigra agglutinin; TPC: total protein of capacitated spermatozoa; TPNC: total protein of non-capacitated spermatozoa; WGA: wheat germ agglutinin; ZP: zona pellucida.
Acknowledgments
We thank Yobana Pérez-Cervera, Ludivine Drougat, Ikram El Yazidi Belkoura and Marlène Mortuaire for technical assistance.
Funding
This work was partially supported by CONACYT, México (grant number 105961-M), scholarship to JBLS (number 233535), Bilateral Cooperation Projects Mexico-France CONACYT-ANUIES-ECOS (grant number M10-A02). The Proteomics facility is funded by the European Community (FEDER), the “Région Nord-Pas de Calais”, the IBISA network, the CNRS, and the University of Lille-Faculté des Sciences et Technologies.
Author information
Authors and Affiliations
Contributions
Direction of the study and established the experimental design: RF, IJM, HGM, ASVE, TL, JCM. Performed the experiments: JBLS, OMP, SFB. All authors analyzed the results, contributed to the finalized manuscript and approved the manuscript.
Corresponding author
Ethics declarations
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publications
Not applicable.
Competing of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
López-Salguero, J.B., Fierro, R., Michalski, JC. et al. Identification of lipid raft glycoproteins obtained from boar spermatozoa. Glycoconj J 37, 499–509 (2020). https://doi.org/10.1007/s10719-020-09924-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-020-09924-0