Abstract
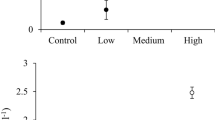
The role of herbivores in regulating aquatic plant dynamics has received growing recognition from researchers and managers. However, the evidence for herbivore impacts on aquatic plants is largely based on short-term exclosure studies conducted within a single plant growing season. Thus, it is unclear how long herbivore impacts on aquatic plant abundance can persist for. We addressed this knowledge gap by testing whether mute swan (Cygnus olor) grazing on lowland river macrophytes could be detected in the following growing season. Furthermore, we investigated the role of seasonal changes in water current speed in limiting the temporal extent of grazing. We found no relationship between swan biomass density in 1 year and aquatic plant cover or biomass in the following spring. No such carry-over effects were detected despite observing high swan biomass densities in the previous year from which we inferred grazing impacts on macrophytes. Seasonal increases in water velocity were associated with reduced grazing pressure as swans abandoned river habitat. Furthermore, our study highlights the role of seasonal changes in water velocity in determining the length of the mute swan grazing season in shallow lowland rivers and thus in limiting the temporal extent of herbivore impacts on aquatic plant abundance.



Similar content being viewed by others
References
Albayrak, I., V. Nikora, O. Miler & M. T. O’Hare, 2014. Flow-plant interactions at leaf, stem and shoot scales: drag, turbulence, and biomechanics. Aquatic Sciences 76: 269–294.
Arnell, N. W., 2003. Relative effects of multi-decadal climatic variability and changes in the mean and variability of climate due to global warming: future streamflows in Britain. Journal of Hydrology 270: 195–213.
Bacon, P. J. & A. E. Coleman, 1986. An analysis of weight changes in the mute swan Cygnus olor. Bird Study 33: 145–158.
Bakker, E. S., J. F. Pagès, R. Arthur & T. Alcoverro, 2016. Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39: 162–179.
Barrat-Segretain, M. H. & D. G. Lemoine, 2007. Can snail herbivory influence the outcome of competition between Elodea species? Aquatic Botany 86: 157–162.
Berrie, A. D., 1992. The chalk-stream environment. Hydrobiologia 248: 3–9.
Birkhead, M. & C. M. Perrins, 1986. The Mute Swan. Croom Helm, London.
Bovee, K. D. & R. Milhouse, 1978. Hydraulic simulation in instream flow studies: theory and techniques. US Fish and Wildlife Service, Office of Biological Services, Fort Collins, Colorado.
Bowes, M. J., J. T. Smith & C. Neal, 2009. The value of high-resolution nutrient monitoring: a case study of the River Frome, Dorset, UK. Journal of Hydrology 378: 82–96.
Chaichana, R., R. Leah & B. Moss, 2011. Seasonal impact of waterfowl on communities of macrophytes in a shallow lake. Aquatic Botany 95: 39–44.
Chambers, P. A., E. E. Prepas, H. R. Hamilton & M. L. Bothwell, 1991. Current velocity and its effect on aquatic macrophytes in flowing waters. Ecological Applications 1: 249–257.
Clausen, P., 2000. Modeling water level influence on habitat choice and food availability for Zostera feeding brent geese Branta bernicla in non-tidal areas. Wildlife Biology 6: 75–87.
Cyr, H. & M. L. Pace, 1993. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature 361: 148–150.
Dawson, F. H., 1976. The annual production of the aquatic macrophyte Ranunculus penicillatus var. calcareus (RW Butcher) CDK Cook. Aquatic Botany 2: 51–73.
Dawson, F. H. & W. N. Robinson, 1984. Submerged macrophytes and the hydraulic roughness of a lowland chalk stream. Verhandlungen International Vereinigung Theoretische Angewandte Limnologie 22: 1944–1948.
Delany, S., 2005. Mute swan Cygnus olor. In Kear, J. (ed.), Ducks, Geese and Swans. Oxford University Press, Oxford: 231–234.
Environment Agency, 2004. The State of England’s Chalk Rivers. A report by the UK Biodiversity Action Plan Steering Group for Chalk Rivers, Environment Agency, Bristol, UK.
Franklin, P., M. Dunbar & P. Whitehead, 2008. Flow controls on lowland river macrophytes: a review. Science of the Total Environment 400: 369–378.
Garner, G., D. M. Hannah, J. P. Sadler & H. G. Orr, 2014. River temperature regimes of England and Wales: spatial patterns, inter-annual variability and climatic sensitivity. Hydrological Processes 28: 5583–5598.
Gayet, G., M. Guillemain, H. Fritz, F. Mesleard, C. Begnis, A. Costiou, G. Body, L. Curtet & J. Broyer, 2011a. Do mute swan (Cygnus olor) grazing, swan residence and fishpond nutrient availability interactively control macrophyte communities? Aquatic Botany 95: 110–116.
Gayet, G., C. Eraud, M. Benmergui, J. Broyer, F. Mesleard, H. Fritz & M. Guillemain, 2011b. Breeding mute swan habitat selection when accounting for detectability: a plastic behaviour consistent with rapidly expanding populations. European Journal of Wildlife Research 57: 1051–1056.
Gordon, N. D., 1992. Stream Hydrology: An Introduction for Ecologists. Wiley, Chichester.
Gurnell, A. M., M. P. Van Oosterhout, B. De Vlieger & J. M. Goodson, 2006. Reach-scale interactions between aquatic plants and physical habitat: River Frome, Dorset. River Research and Applications 22: 667–680.
Gurnell, A. M., J. M. O’Hare, M. T. O’Hare, M. J. Dunbar & P. M. Scarlett, 2010. An exploration of associations between assemblages of aquatic plant morphotypes and channel geomorphological properties within British rivers. Geomorphology 116: 135–144.
Hannaford, J. & G. Buys, 2012. Trends in seasonal river flow regimes in the UK. Journal of Hydrology 475: 158–174.
Harrison, M. D. K., 1985. Report on the assessment of damage to agriculture by mute swans in the Wylye Valley 1984/85. Agricultural Development Advisory Service, Bristol, UK.
Haury, J. & L. G. Aïdara, 1999. Macrophyte cover and standing crop in the River Scorff and its tributaries (Brittany, northwestern France): scale, patterns and process. Hydrobiologia 415: 109–115.
Heck, K. L. & J. F. Valentine, 2006. Plant–herbivore interactions in seagrass meadows. Journal of Experimental Marine Biology and Ecology 330: 420–436.
Hilton, J., M. O’Hare, M. J. Bowes & J. I. Jones, 2006. How green is my river? A new paradigm of eutrophication in rivers. Science of the Total Environment 365: 66–83.
Jermy, T., 1984. Evolution of insect/host plant relationships. American Naturalist 124: 609–630.
Klaassen, M. & B. A. Nolet, 2007. The role of herbivorous water birds in aquatic systems through interactions with aquatic macrophytes, with special reference to the Bewick’s Swan – Fennel Pondweed system. Hydrobiologia 584: 205–213.
Lodge, D. M., 1991. Herbivory on freshwater macrophytes. Aquatic Botany 41: 195–224.
Madsen, J. D., P. A. Chambers, W. F. James, E. W. Koch & D. F. Westlake, 2001. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444: 71–84.
Milchunas, D. G. & W. K. Lauenroth, 1993. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecological Monographs 63: 327–366.
Miler, O., I. Albayrak, V. I. Nikora & M. O’Hare, 2012. Biomechanical properties of aquatic plants and their effects on plant–flow interactions in streams and rivers. Aquatic Sciences 74: 31–44.
Miler, O., I. Albayrak, V. I. Nikora & M. O’Hare, 2014. Biomechanical properties and morphological characteristics of lake and river plants: implications for adaptations to flow conditions. Aquatic Sciences 76: 465–481.
Miller, S. A. & T. A. Crowl, 2006. Effects of common carp (Cyprinus carpio) on macrophytes and invertebrate communities in a shallow lake. Freshwater Biology 51: 85–94.
Mitchell, S. F. & R. T. Wass, 1996. Quantifying herbivory: grazing consumption and interaction strength. Oikos 76: 573–576.
Newman, R. M., 1991. Herbivory and detritivory on freshwater macrophytes by invertebrates: a review. Journal of the North American Benthological Society 10: 89–114.
Nolet, B. A., V. A. Andreev, P. Clausen, M. J. Poot & E. G. Wessel, 2001. Significance of the White Sea as a stopover for Bewick’s swans Cygnus columbianus bewickii in spring. Ibis 143: 63–71.
O’Hare, M. T., R. A. Stillman, J. McDonnell & L. R. Wood, 2007a. Effects of mute swan grazing on a keystone macrophyte. Freshwater Biology 52: 2463–2475.
O’Hare, M. T., K. Hutchinson & R. T. Clarke, 2007b. The drag and reconfiguration experienced by five macrophytes from a lowland river. Aquatic Botany 86: 253–259.
O’Hare M. T., P. Scarlett, P. Henville, T. Ryaba, C. Cailes & J. Newman, 2008. Variability in Manning’s n estimates for vegetated rivers. Core Site Study. Intra- and Inter- Annual Variability. An Aquatic Plant Management Group Report. Centre for Ecology & Hydrology, UK.
Porteus, T. A., M. J. Short, J. C. Reynolds, D. N. Stubbing, S. M. Richardson & N. J. Aebischer, 2008. The impact of grazing by mute swans (Cygnus olor) on the biomass of chalk stream macrophytes. Unpublished report to the Environment Agency. Game and Wildlife Conservation Trust, Hampshire, UK
R Development Core Team, 2015. R: a language and environment for statistical computing. [3.1.2]. R Foundation for Statistical Computing, Vienna, Austria.
Riis, T. & B. J. Biggs, 2003. Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnology & Oceanography 48: 1488–1497.
Royan, A., D. M. Hannah, S. J. Reynolds, D. G. Noble & J. P. Sadler, 2013. Avian community responses to variability in river hydrology. PLoS One 8: e83221.
Royan, A., C. Prudhomme, D. M. Hannah, S. J. Reynolds, D. G. Noble & J. P. Sadler, 2015. Climate-induced changes in river flow regimes will alter future bird distributions. Ecosphere 6: 50.
Søndergaard, M., L. Bruun, T. Lauridsen, E. Jeppesen & T. V. Madsen, 1996. The impact of grazing waterfowl on submerged macrophytes: in situ experiments in a shallow eutrophic lake. Aquatic Botany 53: 73–84.
Stillman, R. A., K. A. Wood, W. Gilkerson, E. Elkinton, J. M. Black, D. H. Ward & M. Petrie, 2015. Predicting effects of environmental change on a migratory herbivore. Ecosphere 6: 114.
Trump, D. P., D. A. Stone, C. F. Coombs & C. J. Feare, 1994. Mute swans in the Wylye Valley: population dynamics and habitat use. International Journal of Pest Management 40: 88–93.
Usherwood, J. R., A. R. Ennos & D. J. Ball, 1997. Mechanical and anatomical adaptations in terrestrial and aquatic buttercups to their respective environments. Journal of Experimental Botany 48: 1469–1475.
van der Wal, J. E., M. Dorenbosch, A. K. Immers, C. Vidal Forteza, J. J. Geurts, E. T. H. M. Peeters, B. Koese & E. S. Bakker, 2013. Invasive crayfish threaten the development of submerged macrophytes in lake restoration. PLoS One 8: e78579.
Vaughan, I. P., D. G. Noble & S. J. Ormerod, 2007. Combining surveys of river habitats and river birds to appraise riverine hydromorphology. Freshwater Biology 52: 2270–2284.
Wass, R. & S. F. Mitchell, 1998. What do herbivore exclusion experiments tell us? An investigation using black swans (Cygnus atratus Latham) and filamentous algae in a shallow lake. In Jeppesen, E., M. Søndergaard, M. Søndergaard & K. Christoffersen (eds), The Structuring Role of Submerged Macrophytes in Lakes. Springer, New York: 282–289.
Watola, G. V., D. A. Stone, G. C. Smith, G. J. Forrester, A. E. Coleman, J. T. Coleman, M. J. Goulding, K. A. Robinson & T. P. Milsom, 2003. Analyses of two mute swan populations and the effects of clutch reduction: implications for population management. Journal of Applied Ecology 40: 565–579.
Webb, B. W., P. D. Clack & D. E. Walling, 2003. Water–air temperature relationships in a Devon river system and the role of flow. Hydrological Processes 17: 3069–3084.
Whittaker, J. B., 1982. The effect of grazing by a chrysomelid beetle, Gastrophysa viridula, on growth and survival of Rumex crispus on a shingle bank. Journal of Ecology 70: 291–296.
Wilby, R. L., 2006. When and where might climate change be detectable in UK river flows? Geophysical Research Letters 33: L19407.
Wood, K. A., R. A. Stillman, R. T. Clarke, F. Daunt & M. T. O’Hare, 2012a. The impact of waterfowl herbivory on plant standing crop: a meta-analysis. Hydrobiologia 686: 157–167.
Wood, K. A., R. A. Stillman, R. T. Clarke, F. Daunt & M. T. O’Hare, 2012b. Understanding plant community responses to combinations of biotic and abiotic factors in different phases of the plant growth cycle. PLoS One 7: e49824.
Wood, K. A., R. A. Stillman, F. Daunt & M. T. O’Hare, 2012c. An individual-based model of swan-macrophyte conflicts on a chalk river. In Boon, P. J. & P. J. Raven (eds), River Conservation and Management. Wiley-Blackwell, Chichester: 339–343.
Wood, K. A., R. A. Stillman, R. T. Clarke, F. Daunt & M. T. O’Hare, 2012d. Measuring submerged macrophyte standing crop in shallow rivers: a test of methodology. Aquatic Botany 102: 28–33.
Wood, K. A., R. A. Stillman, D. Wheeler, S. Groves, C. Hambly, J. R. Speakman, F. Daunt & M. T. O’Hare, 2013a. Go with the flow: water velocity regulates herbivore foraging decisions in river catchments. Oikos 122: 1720–1729.
Wood, K. A., R. A. Stillman, T. Coombs, C. McDonald, F. Daunt & M. T. O’Hare, 2013b. The role of season and social grouping on habitat use by mute swans (Cygnus olor) in a lowland river catchment. Bird Study 60: 229–237.
Wood, K. A., R. A. Stillman, F. Daunt & M. T. O’Hare, 2015. The swan grazing conflict in chalk rivers. In Redpath, S. M., R. J. Gutierrez, K. A. Wood & J. C. Young (eds), Conflicts in Conservation: Navigating Towards Solutions. Cambridge University Press, Cambridge: 134–136.
Wood, K. A., M. T. O’Hare, C. McDonald, K. R. Searle, F. Daunt & R. A. Stillman, 2016. Herbivore regulation of plant abundance in aquatic ecosystems. Biological Reviews. doi:10.1111/brv.12272
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14.
Acknowledgments
We thank the Freshwater Biological Association and the other riparian landowners for allowing access to the study river reaches and for logistical support. The Environment Agency provided daily water discharge data. Pete Scarlett and Lucy Mulholland kindly assisted with macrophyte species identification and data collection, respectively. Two anonymous reviewers provided valuable feedback on an earlier version of this manuscript. This study was funded by the Natural Environment Research Council (NERC) through a Centre for Ecology & Hydrology Algorithm studentship awarded to KAW (NEC3579).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. T. O’Hare, F. C. Aguiar, E. S. Bakker & K. A. Wood / Plants in Aquatic Systems – a 21st Century Perspective
Rights and permissions
About this article
Cite this article
Wood, K.A., Stillman, R.A., Clarke, R.T. et al. Water velocity limits the temporal extent of herbivore effects on aquatic plants in a lowland river. Hydrobiologia 812, 45–55 (2018). https://doi.org/10.1007/s10750-016-2744-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2744-4