Abstract
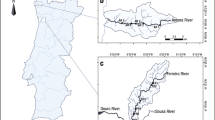
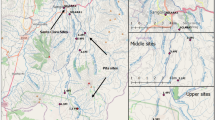
The assessment of ecological integrity of river systems is multidisciplinary and necessary for effective river management. The objectives of this study were (i) to characterize the spatial and temporal distribution of macroinvertebrate and algae community assemblages; (ii) to determine the environmental variables that affect assemblage distributions; and (iii) to determine the suitability of the selected bioindicators in relation to environmental conditions. Two agriculturally influenced coastal rivers, in the southern Cape Province, South Africa, provided case studies. Wet and dry season’s results indicated that minimally impacted sites were associated with pollution-sensitive macroinvertebrate and algal taxa with increased habitat scores. These sites were dominated by diatoms and macroinvertebrates indicative of low electrical conductivity (12–16 mS m−1), pH (4–5), and alkalinity (0.5–2.1 mg l−1). A positive correlation between nitrogen and phosphorus and river flow regime occurred at agriculturally impacted sites and algal taxa changes were driven by nutrient enrichment. Macroinvertebrates were indicative of habitat integrity and river condition while diatoms were indicative of pH and electrical conductivity. The benthic filamentous algae were indicative of increased nutrients and alkalinity. Results suggest that the full consortium of algae and macroinvertebrates be used as bioindicators for ecological integrity assessments in short, coastal rivers, which have application to rivers generally.






Similar content being viewed by others
References
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods, Plymouth, UK, PRIMER-E.
APHA, 2006. Standard Methods for Examination of Water and Wastewater, 20th ed. American Public Health Association, Washington, DC.
Biggs, B. J. F., 2000. Eutrophication of streams and rivers: dissolved nutrients—chlorophyll relationships for benthic algae. Journal of North American Benthological Society 19: 17–31.
Biggs, B. J. F. & G. M. Price, 1987. A survey of filamentous algal proliferations in New Zealand rivers. New Zealand Journal of Marine and Freshwater Research 21: 175–191.
Biggs, B. J. F. & R. A. Smith, 2002. Taxonomic richness of stream benthic algae: effects of flood disturbance and nutrients. Limnology and Oceanography 47: 1175–1186.
Blettler, M. C. M., M. L. Amsler, E. G. Eberle, R. Szupiany, F. G. Latosinski, E. Abrial, P. J. Oberholster, L. A. Espinola, A. Paira, A. Poza & R. A. Capitulo, 2016. Linking hydro-morphology with invertebrate ecology in diverse morphological units of a large river-floodplain system. Water Resources Research 52: 9495–9510.
Buffington, J. M. & D. R. Montgomery, 2013. 9.36 Geomorphic classification of rivers. In Shroder, J. F. (ed.), Treatise on Geomorphology. Academic Press, San Diego: 730–767.
Chessman, B., I. Growns, J. Currey & N. Plunkett-Cole, 1999. Predicting diatom communities at the genus level for the rapid biological assessment of rivers. Freshwater Biology 41: 317–331.
Chételat, J., F. R. Pick, A. Morin & P. B. Hamilton, 1999. Periphyton biomass and community composition in rivers of different nutrient status. Canadian Journal of Fisheries and Aquatic Sciences 56: 560–569.
Chon, T. S., X. Qu, W. S. Cho, H. J. Hwang, H. Tang, Y. Liu, J. H. Choi, M. Jung, B. S. Chung, H. Y. Lee & Y. R. Chung, 2013. Evaluation of stream ecosystem health and species association based on multi-taxa (benthic macroinvertebrates, algae, and microorganisms) patterning with different levels of pollution. Ecological Informatics 17: 58–72.
Clarke, K. R. & R. N. Gorley, 2006. Primer V6: User Manual/Tutorial, Plymouth, UK.
Clarke, K. R. & R. M. Warwick, 2001. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed. PRIMER-E, Ltd., Plymouth.
Dela-Cruz, J., T. I. M. Pritchard, G. Gordon & P. Ajani, 2006. The use of periphytic diatoms as a means of assessing impacts of point source inorganic nutrient pollution in south-eastern Australia. Freshwater Biology 51: 951–972.
Descy, J. P. & M. Coste, 1991. A test of methods for assessing water quality based on diatoms. Verhandlungen des Internationalen Verein Limnologie 24: 2112–2116.
Dewson, Z. S., A. B. James & R. G. Death, 2007. A review of the consequences of decreased flow for instream habitat and macroinvertebrates. Journal of the North American Benthological Society 26: 401–415.
Dickens, C. W. S. & P. M. Graham, 2002. The South African Scoring System (SASS) version 5 rapid bioassessment method for rivers. African Journal of Aquatic Science 27: 1–10.
Dodds, W. K., V. H. Smith & K. Lohman, 2002. Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Canadian Journal of Fisheries and Aquatic Sciences 59: 865–874.
Douterelo, I., E. Perona & P. Mateo, 2004. Use of cyanobacteria to assess water quality in running waters. Environmental Pollution 127: 377–384.
DWAF, 1996a. South African Water Quality Guidelines. Volume 1. Domestic Water Use, Vol. 1, 2nd ed. Department of Water Affairs and Forestry, Pretoria.
DWAF, 1996b. South African Water Quality Guidelines (2nd Edition). Volume 7: Aquatic Ecosystems. DWAF, Pretoria.
Eady, B. R., T. R. Hill & N. A. Rivers-Moore, 2014. Shifts in aquatic macroinvertebrate community structure in response to perenniality, southern Cape, South Africa. Journal of Freshwater Ecology 29: 475–490.
Elosegi, A., J. Díez & M. Mutz, 2010. Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiologia 657: 199–215.
Ewart-Smith, J. & J. King, 2012. The Relationship Between Periphyton, Flow and Nutrient Status in South-Western Cape Foothill Rivers and the Implications for Management, Water Research Commission Report No. 1676/1/12, Pretoria, South Africa.
Ewart-Smith, J. L., 2012. The Relationship Between Periphyton, Flow and Nutrients in Foothill Rivers of the South-Western Cape. Department of Zoology, University of Cape Town, Cape Town: 1–285.
Feminella, J. W. & C. P. Hawkins, 1995. Interactions between stream herbivores and periphyton: a quantitative analysis of past experiments. Journal of North American Benthological Society 14: 465–509.
Figueroa-Nieves, D., T. V. Royer & M. B. David, 2006. Controls on chlorophyll-a in nutrient-rich agricultural streams in Illinois, USA. Hydrobiologia 568: 287–298.
Gökçe, D., 2016. Algae as an indicator of water quality. In Dhanasekaran, D. (ed.), Algae – Organisms for Imminent Biotechnology. InTech, Rijeka: 1–18.
Harding, W. R., C. G. M. Archibald & J. C. Taylor, 2005. The relevance of diatoms for water quality assessment in South Africa: a position paper. Water SA 31: 41–46.
Hawkins, C. P., J. N. Hogue, L. M. Decker & J. W. Feminella, 1997. Channel morphology, water temperature, and assemblage structure of stream insects. Journal of North American Benthological Society 16: 728–749.
Holt, E. A. & S. W. Miller, 2011. Bioindicators: using organisms to measure environmental impacts. Nature Education Knowledge 3: 8.
Jacobson, R. B., 2013. 12.2 riverine habitat dynamics. In Shroder, J. F. (ed.), Treatise on Geomorphology. Academic Press, San Diego: 6–19.
King, J. M. & D. M. Schael, 2001. Assessing the Ecological Relevance of a Spatially-Nested Geomorphological Hierarchy for River Management, Water Research Commission Report No. 754/1/01, Pretoria, South Africa.
Komarek, J. & K. Anagnostidis, 1999. Cyanoprokaryota: Chroococcales. In Ettl, H., H. H. Gartner & D. Mollenhauer (eds), Susswasserflora von Mitteleuropa 19/1. Gustav Fischer, Stuttgart.
Komarek, J. & K. Anagnostidis, 2005. Cyanoprokaryota: Oscillatoriales. In Budel, B., G. Gartner, L. Krienitz & M. Schager (eds), Susswasserflora von Mitteleuropa 19/2. Elsevier, Munchen.
Kutka, F. J. & C. Richards, 1996. Relating diatom assemblage structure to stream habitat quality. Journal of the North American Benthological Society 15: 469–480.
Li, L., B. Zheng & L. Liu, 2010. Biomonitoring and bioindicators used for river ecosystems: definitions, approaches and trends. Procedia Environmental Sciences 2: 1510–1524.
Lubke, R. & I. de Moor, 1998. Field Guide to the Eastern and Southern Cape Coasts. University of Cape Town Press, Cape Town.
Marks, J. C. & R. L. Lowe, 1989. The independent and interactive effects of snail grazing and nutrient enrichment on structuring periphyton communities. Hydrobiologia 185: 9–17.
Márquez, J. A., L. Cibils, R. E. Principe & R. J. Albariño, 2015. Stream macroinvertebrate communities change with grassland afforestation in central Argentina. Limnologica - Ecology and Management of Inland Waters 53: 17–25.
McMillan, P. H., 1998. An Integrated Habitat Assessment System (IHAS v2), for the Rapid Biological Assessment of Rivers and Streams. CSIR, Pretoria.
Merritt, R. W. & K. W. Cummins, 1996. An Introduction to the Aquatic Insects of North America, 3rd ed. Kendal/Hunt, Dubuque.
Munn, M. D., L. L. Osborne & M. J. Wiley, 1989. Factors influencing periphyton growth in agricultural streams of central Illinois. Hydrobiologia 174: 89–97.
Oberholster, P. J., 2011. Using epilithic filamentous green algae communities as indicators of water quality in the headwaters of three South African river systems during high and medium flow periods. In Kattel, G. (ed.), Zooplankton and Phytoplankton. Nova Science Publishers Inc, New York: 1–16.
Oberholster, P. J. & A. R. de Klerk, 2014. Aquatic ecosystems in the coal mining landscape of the Upper Olifants River, and the way forward. 21st Century challenges to the southern African coal Sector, The Southern African Institute of Mining and Metallurgy, Johannesburg: 237–251.
Oberholster, P. J., V. S. Somerset, J. C. Truter & A.-M. Botha, 2016. The interplay between environmental conditions and filamentous algae mat formation in two agricultural influenced South African rivers. River Research and Applications 33: 388–402.
Oberholster, P. J., A. M. Botha, L. Hill & W. F. Strydom, 2017. River catchment responses to anthropogenic acidification in relationship with sewage effluent: an ecotoxicology screening application. Chemosphere 189: 407–417.
Petersen, C. R., N. Z. Jovanovic, D. C. Le Maitre & M. C. Grenfell, 2017. Effects of land use change on streamflow and stream water quality of a coastal catchment. Water SA 43: 551–564.
Porra, R. J., W. A. Thompson & P. E. Kriedemann, 1989. Determination of accurate extinction coefficient and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochimica et Biophysica Acta 975: 384–394.
Porter, S. D., D. K. Mueller, N. E. Spahr, M. D. Munn & N. M. Dubrovsky, 2008. Efficacy of algal metrics for assessing nutrient and organic enrichment in flowing waters. Freshwater Biology 53: 1036–1054.
Qu, X., H. Zhang, M. Zhang, M. Liu, Y. Yu, Y. Xie & W. Peng, 2016. Application of multiple biological indices for river health assessment in northeastern China. Annales de Limnologie-International Journal of Limnology 52: 75–89.
Resh, V. H., 2008. Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environmental Monitoring and Assessment 138: 131–138.
Rowntree, K. M., R. A. Wadeson & J. O’Keefe, 2000. The development of a Geomorphological classification system for the longitudinal zonation of South African rivers. South African Geographical Journal 82: 163–172.
Russell, I. A., 2013. Spatio-temporal variability of surface water quality parameters in a South African estuarine lake system. African Journal of Aquatic Science 38: 53–66.
Santoul, F., J. Cayrou, S. Mastrorillo & R. Céréghino, 2005. Spatial patterns of the biological traits of freshwater fish communities in south-west France. Journal of Fish Biology 66: 301–314.
Schael, D. M., 2005. Distributions of physical habitats and benthic macroinvertebrates in Western Cape headwater streams at multiple spatial and temporal scales. University of Cape Town, Cape Town: 1–239.
Schneider, S. C. & E.-A. LindstrØm, 2011. The periphyton index of trophic status PIT: a new eutrophication metric based on non-diatomaceous benthic algae in Nordic rivers. Hydrobiologia 665: 143–155.
Smith, J., M. J. Samways & S. Taylor, 2007. Assessing riparian quality using two complementary sets of bioindicators. Biodiversity and Conservation 16: 2695–2713.
Stevenson, R. J. & L. L. Bahls, 1999. Periphyton protocols. In Barbour, M. T., J. Gerritren, B. D. Snyder & J. B. Stribling (eds), Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish 2ndEPA 841-B-99-002 edn. United States Environmental Protection Agency, Washington, DC: 6–22.
Summers, J. S., 2008. Assessment of filamentous algae in the Greenbrier River and other West Virginia streams. West Virginia Department of Environmental Protection, West Virginia.
Taylor, J. C., W. R. Harding & C. G. M. Archibald, 2007a. An Illustrated Guide to Some Comman Diatom Species from South Africa, Water Research Commission Report No. TT 282/07, Pretoria, South Africa.
Taylor, J. C., J. Prygiel, A. Vosloo, P. A. de la Rey & L. van Rensburg, 2007b. Can diatom-based pollution indices be used for biomonitoring in South Africa? A case study of the Crocodile West and Marico water management area. Hydrobiologia 592: 455–464.
Thorp, J. H., M. C. Thoms & M. D. Delong, 2006. The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Research and Applications 22: 123–147.
Van Vuuren, S., J. C. Taylor, A. Gerber & C. Van Ginkel, 2006. Easy Identification of the Most Common Freshwater Algae. Department of Water Affairs and Forestry, Pretoria.
Wehr, J. D. & R. G. Sheath, 2003. Freshwater habitats of algae. In Wehr, J. D., R. G. Sheath & P. Kociolek (eds), Freshwater Algae of North America: Ecology and Classification. Academic Press, San Diego, CA: 11–57.
Wolman, M. G., 1954. A method of sampling coarse river-bed material EOS. Transactions of the American Geophysical Union 35: 951–956.
Wurts, W. & R. Durborow, 1992. Interaction of carbon dioxide, pH, alkalinity and hardness in fish ponds, United States Department of Agriculture, Southern Regional Aquaculture Centre (SRAC), Publication No. 464.
Acknowledgments
The authors acknowledge financial support from the CSIR’s Parliamentary Grant funding (Project Number ECRS002), National Research Foundation (Grant Number: 84209), the Department of Science and Technology (DST) (Project Number ECRS005), and the Water Research Commission (WRC) (2013–2016). Thanks to colleagues at the CSIR for assisting with fieldwork and for valuable comments on earlier drafts. We also want to thank the landowners in the Wilderness area for their support. The authors want to thank the anonymous referees for critically reviewing the manuscript and suggesting changes. Data may be obtained from the CSIR Research Space data repository (https://researchspace.csir.co.za/dspace/handle/10204/8989). Any additional data may be obtained from the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: David Philip Hamilton
Rights and permissions
About this article
Cite this article
Petersen, C.R., Jovanovic, N.Z., Grenfell, M.C. et al. Responses of aquatic communities to physical and chemical parameters in agriculturally impacted coastal river systems. Hydrobiologia 813, 157–175 (2018). https://doi.org/10.1007/s10750-018-3518-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3518-y