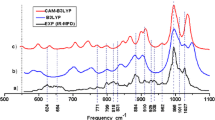
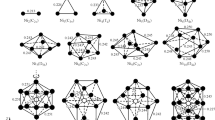
Structural models were designed and spectral characteristics were computed based on DFT calculations of uranium monoxide clusters (UO)2, (UO)4, (UO)6, and (UO)9. Spectral features that were characteristic of the cluster formation process were identified. The uranium oxidation state was close to 3 in the clusters (UO)2, (UO)4, and (UO)6. The vibrational frequencies decreased monotonically in the sequence (UO)2 → (UO)4 → (UO)6 because of decreasing electron density in each of the UO bonds with the growing complexity of the clusters. The uranium oxidation state was close to 4 in the cluster (UO)9. This led to a strengthening of the bonds and an increase in the frequency of the strongest band in the IR spectrum.
Similar content being viewed by others
References
L. R. Morss, N. M. Edelstein, and J. Fuger (Eds.), The Chemistry of the Actinide and Transactinide Elements, 4th edn., Springer, Dordrecht (2006).
V. F. Petrunin and A. V. Fedotov, Scientific Session of MIFI [in Russian], Moscow, 9, 198–199 (2006).
S. D. Gabelnick, G. T. Reedy, and M. G. Chasanov, J. Chem. Phys., 58, 4468–4475 (1973).
S. D. Gabelnick, G. T. Reedy, and M. G. Chasanov, J. Chem. Phys., 59, 6397–6404 (1973).
R. D. Hunt and L. Andrews, J. Chem. Phys., 98, 3690–3696 (1993).
M. Zhou, L. Andrews, N. Ismail, and C. Marsden, J. Phys. Chem. A, 104, 5495–5502 (2000).
A. Barnes, W. J. Orville-Thomas, R. Gaufres, and A. Muller (Eds.), Matrix Isolation Spectroscopy, Springer (1981).
J. Li, B. E. Bursten, L. Andrews, and C. J. Marsden, J. Am. Chem. Soc., 126, 3424–3425 (2004).
J. Han, V. Goncharov, L. A. Kaledin, A. V. Komissarov, and M. C. Heaven, J. Chem. Phys., 120, 5155–5163 (2004).
L. Gagliardi, M. C. Heaven, J. W. Krogh, and B. O. Roos, J. Am. Chem. Soc., 127, 86–91 (2005).
P. Li, T.-T. Jia, T. Gao, and G. Li, Chin. Phys. B, 21, 043301 (2012).
M. B. Shundalau, A. P. Zajogin, A. I. Komiak, A. A. Sokolsky, and D. S. Umreiko, J. Spectrosc. Dyn., 2, 19 (2012).
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery, J. Comput. Chem., 14, 1347–1363 (1993).
B. M. Bode and M. S. Gordon, J. Mol. Graphics Modell., 16, 133–138 (1998).
L. J. Farrugia, J. Appl. Crystallogr., 30, 565 (1997).
L. R. Kahn, P. J. Hay, and R. D. Cowan, J. Chem. Phys., 68, 2386–2397 (1978).
T. H. Dunning, Jr., J. Chem. Phys., 90, 1007–1023 (1989).
D. Feller, J. Comput. Chem., 17, 1571–1586 (1996).
K. L. Schuchardt, B. T. Didier, T. Elsethagan, L. Sun, V. Gurumoorthi, J. Chase, J. Li, and T. L. Windus, J. Chem. Inf. Model., 47, 1045–1052 (2007).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 37, 785–789 (1988).
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch, J. Phys. Chem., 98, 11623–11627 (1994).
L. A. Kaldein and M. C. Heaven, J. Mol. Spectrosc., 185, 1–7 (1997).
M. B. Shundalov, A. I. Komyak, A. P. Zazhogin, and D. S. Umreiko, Zh. Prikl. Spektrosk., 79, No. 1, 27–36 (2012).
M. B. Shundalau, A. I. Komiak, A. P. Zajogin, and D. S. Umreiko, J. Spectrosc. Dyn., 3, 4 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 80, No. 4, pp. 545–550, July–August, 2013.
Rights and permissions
About this article
Cite this article
Shundalau, M.B., Umreiko, D.S., Zazhogin, A.P. et al. Modeling IR spectra of uranium monoxide clusters. J Appl Spectrosc 80, 530–535 (2013). https://doi.org/10.1007/s10812-013-9800-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-013-9800-x