Abstract
Purpose
To develop a consensus statement for left atrial appendage occlusion (LAAO) in Asian-Pacific patients with non-valvular atrial fibrillation (NVAF) at risk of ischemic stroke. The need for such a region-specific consensus was indicated by the relative paucity of clinical evidence for LAAO and oral anticoagulation therapy obtained in Asian-Pacific populations and the specific stroke and bleeding characteristics of this population.
Methods
Consensus was developed by discussion and evaluation of available evidence and expert opinions during a 2-day meeting attended by clinical experts from the Asian-Pacific regions.
Results
The consensus statement arrived at provides recommendations based on available evidence and expert opinions regarding LAAO in Asian-Pacific patients. Gaps in the evidence and other areas requiring further research were identified.
Conclusion
LAAO is an alternative device-based therapy in carefully selected patients with NVAF at risk of ischemic stroke. However, evidence for LAAO is primarily obtained from Caucasian populations, and data on LAAO in Asian-Pacific patients are scarce. While the present consensus statement addresses several therapy-related aspects based on careful interpretation of available evidence and expert opinions, other areas require additional evidence derived from Asian-Pacific populations.
Similar content being viewed by others
1 Introduction
Oral anticoagulation (OAC) therapy using warfarin has been demonstrated to significantly reduce the risk for ischemic stroke in patients with non-valvular atrial fibrillation (NVAF) compared with antiplatelet therapy (APT) [1,2,3]. However, its use is associated with a substantial risk for major and life-threatening bleeding. Non-vitamin K antagonist oral anticoagulants (NOACs) provide similar or better stroke prevention than warfarin with reduced bleeding risks. Nevertheless, OAC and NOAC are still associated with clinically significant bleeding, in particular intracranial hemorrhage and gastrointestinal bleeding [4,5,6,7,8]. This complicates clinical decision-making, particularly in patients with multiple risk factors for bleeding and ischemic stroke, such as hypertension, prior major bleeding or stroke, concomitant intake of antiplatelet drugs, renal and hepatic dysfunction and advanced age.
To overcome OAC-associated bleeding complications, device-based alternatives have been developed with the potential to eliminate the need for long-term anticoagulation. The left atrial appendage (LAA) is the predominant location of thrombus formation in patients with NVAF [9], as was recently confirmed by a transesophageal echocardiography (TEE) study that found an LAA thrombus in 6.13% of 1420 patients referred to cardioversion for NVAF or atrial flutter [10]. Closure of this source of cardiac thrombi by percutaneous left atrial appendage occlusion (LAAO) potentially reduces the risk of cardioembolic stroke. The proof of this concept was provided by the randomized controlled PROTECT AF and PREVAIL trials, demonstrating effective stroke prevention by LAAO in NVAF patients eligible for warfarin [11, 12]. Large multicenter prospective registries [13,14,15] have provided further clinical evidence for LAAO in patients with and without contraindications to long-term OAC. Results of these studies typically show a risk reduction for ischemic stroke as well as for major bleeding, compared with expected rates based on the patients’ risk factors. Several randomized controlled trials of LAAO are currently ongoing, primarily enrolling patients not suitable for long-term (N)OAC, including the ASAP-TOO study [16], the STROKE-CLOSE study [17] assessing the benefit of LAAO in NVAF patients with a recent intracerebral hemorrhage, and the CLOSURE-AF [18] trial enrolling patients with a high bleeding risk (e.g., HAS-BLED ≥ 3) or a history of major bleeding.
While a bleeding risk accumulating over time is the major safety concern associated with long-term OAC, the predominant risks associated with LAAO are related to the implantation of the device and the early post-implantation period. Major procedure-related complications of LAAO include pericardial effusion and tamponade and major procedural bleeding, typically related to vascular access complications. The most significant device-related complications include device-related thrombus (DRT) and device embolization.
Current clinical practice regarding post-implant antithrombotic therapy is highly heterogeneous and poorly supported by data from prospective clinical studies [19]. Despite the use of warfarin post-implantation in the randomized controlled trials, aspirin and clopidogrel are the most frequently prescribed during the first 6 months after implantation, and heparin and NOAC are specifically used to resolve DRT. The typical duration of antithrombotic therapy ranges between 45 days and 6 months after implantation, but only limited data is available to guide decision-making regarding the most optimal therapy duration [20]. The increased bleeding risk in Asian-Pacific patients and the paucity of data from this population leave the physician with many uncertainties regarding this aspect.
Current guidelines [21, 22] include class IIb/level B recommendations for LAAO in NVAF patients, primarily based on evidence from patients eligible for (N)OACs. A recently updated European consensus statement [23] recommends LAAO to be considered for patients with absolute contraindications for long-term (N)OAC or patients with a high risk for bleeding on NOACs that outweighs their demonstrated benefits in the prevention of ischemic stroke. Additionally, LAAO may be considered for non-compliant patients (when attempts to resolve non-compliance were unsuccessful) and for those who had a stroke despite adequate oral anticoagulation. While physicians gain further experience with this therapy, LAAO remains a technically challenging intervention, requiring state-of-the-art imaging techniques and a well-trained staff, prepared to identify and resolve procedural complications [24, 25].
The most prominent uncertainties regarding LAAO include the effectiveness in patients contraindicated to long-term (N)OAC and benefits of LAAO versus contemporary NOAC therapy [23].
NVAF and associated stroke present a considerable burden to public health and healthcare costs in the Asian-Pacific region. In China, the prevalence of atrial fibrillation and of associated stroke increased 20-fold and 13-fold, respectively, between 2001 and 2011 [26]. However, only 32% were taking aspirin, and 4% were using warfarin. LAAO may be one of the potential answers to this major healthcare burden, as was underlined by the prospective observational WASP study, conducted in centers across Asia, Australia, and the Middle East [27]. Compared with non-Asian patients, LAAO achieved a more pronounced reduction in major bleeding and in the composite of ischemic stroke, transient ischemic attack, and systemic embolism in Asians. Besides this study and some smaller single-center studies, most data from randomized controlled trials and large prospective registries on LAAO has been collected outside the Asian-Pacific region, involving primarily Caucasian patients. As a result, this evidence and the guidelines and consensus statements derived from it may insufficiently address specific aspects applicable to Asian-Pacific patients. In large randomized anticoagulation trials for stroke prevention in atrial fibrillation, anticoagulated Asian patients showed higher ischemic stroke risks as well as more frequent major bleeding on warfarin, compared with Caucasians [28]. This was observed for major, life-threatening, gastrointestinal, intracranial, and minor bleeding. On the other hand, in the RE-LY trial, Asian patients taking a 150 mg daily dose of dabigatran had a net clinical benefit (composite of stroke, systemic embolism, and major bleeding) similar to non-Asian patients [29], and reduction in major bleeding by apixaban versus warfarin was most pronounced in Asian-Pacific patients [7] compared with other ethnic groups. Overall, non-warfarin oral anticoagulants (NOACs) are preferred for stroke prevention in Asian patients over warfarin, primarily based on safety rather than efficacy [29, 30]. In conclusion, the clinical evidence and benefit-risk profile of stroke prevention therapies, primarily established in Caucasian cohorts, may not be directly extrapolated to Asian-Pacific patients.
Given the relative deficiency of comprehensive evidence for LAAO in the Asian-Pacific population, a group of clinical experts convened during the Asian-Pacific Heart & Brain Summit (July 13/14, 2019; Bangkok, Thailand) with the objective to develop an expert consensus statement for LAAO in Asian-Pacific NVAF patients. While this consensus was aimed to address gaps in the general evidence, consensus statements were developed to account for aspects specific for the Asian-Pacific population, as far as meaningful and applicable.
Consensus is presented per distinct topic, by using various consensus levels and corresponding symbols, as explained in Table 1.
2 Consensus topics
2.1 What is the clinical evidence specifically required for LAAO in Asian-Pacific patients?
Large randomized controlled trials [11, 12] and prospective registries [13,14,15] have provided comprehensive clinical evidence for LAAO, primarily in Caucasian populations and in comparison with warfarin therapy. However, aspects specific to the Asian-Pacific population as well as direct comparison with NOACs have been insufficiently addressed. Unanswered questions with regard to LAAO in Asian-Pacific patients are mainly raised by the specific bleeding risk in this population [29, 30], the superior stroke prevention of NOACs over warfarin [31, 32], and off-label underdosing of NOACs in Asian-Pacific patients due to perceived bleeding risks [33]. Therefore, regional and/or population data sets are required to assess the benefit-risk profile of LAAO in these patients compared with the clinical practice of NOAC use. Particular aspects to be studied are listed in Table 2.
2.2 Balancing stroke and bleeding risk in NVAF patients
In patients with NVAF, the same risk factors indicating anticoagulation for stroke prevention also predict an elevated bleeding risk, associated with anticoagulation (i.e., hypertension, prior stroke, and age). While this raises a dilemma for treatment of NVAF patients in general, the increased bleeding risk in Asian-Pacific patients may even further complicate clinical decision-making. Compared with non-Asian patients, Asians have higher risks of major and gastrointestinal bleeding [28, 30], and the relatively high prevalence of intracranial macro- and microangiopathy in Asian patients may contribute to their higher risk of warfarin-associated intracranial bleeding [34,35,36]. The HAS-BLED score is a well-established assessment for bleeding risks, but an individualized approach is required with regard to patient-specific risk factors not addressed by the HAS-BLED assessment. While NOACs are demonstrated to provide at least non-inferior stroke prevention compared with warfarin, they may still cause major bleeding, for which reason Asian-Pacific patients may be undertreated [37] or frequently receive reduced doses [38, 39]. However, particularly off-label underdosing of NOACs is associated with increased cardiovascular hospitalization, while it does not significantly reduce the most feared bleeding complications [40]. Therefore, for eligible patients with limited bleeding risks, the use of NOACs dosed according to established recommendations is the preferred therapy. Moreover, LAAO should not be offered as an equally safe and effective therapy to these patients.
The CHA2DS2-VASc score is the preferred method to assess the risk for ischemic stroke in NVAF patients. However, there are indications that Asian patients have a lower age threshold for stroke compared with non-Asians [28], e.g., as suggested by better performance in Asian AF patients of a modified CHA2DS2-VASc score using an age of 50 years instead of the original 65 years as a cut-off point [41]. Guidelines [21, 22] recommend (N)OAC therapy for male and female NVAF patients with ≥ 2 and ≥ 3 risk factors included in the CHA2DS2-VASc score, respectively. In these patients, and especially in those with borderline risks, the use of anticoagulant therapy should be based on an individualized decision, accounting for contraindications and bleeding risk factors and with correction of modifiable risk factors.
Consensus aspects related to (N)OAC management and risk assessment are summarized in Table 3.
2.3 Patient selection and indications for LAAO
The indication scheme presented in the recently update EHRA/EAPCI consensus statement on LAAO [23] provides guidance for clinical decision-making with respect to LAAO indications for Asian-Pacific patients. According to this scheme, LAAO may primarily be considered for patients with absolute contraindications for long-term oral anticoagulation therapy, patients who are unwilling to take long-term anticoagulation, those with an elevated bleeding risk on anticoagulation, or with individual and specific risk for stroke, such as inefficient OAC (“stroke on warfarin”). Consensus on indications is summarized in Table 4. Among these indications, LAAO in patients not eligible to any antithrombotic therapy remains to be applied with caution and strictly based on an individual approach, given the routine to provide dual or single APT for variable time after device implantation. In patients who are ineligible for even a short period of APT therapy, the benefit-risk profile of LAAO should be evaluated against that of epicardial approaches such as (minimal invasive, thoracoscopic) surgical closure or use of the LARIAT device [23]. LAAO may be considered as an alternative to uninterrupted OAC to overcome the increased risk of ischemic stroke associated with electrical isolation of the LAA [42]. While the rationale of stroke prevention by LAA exclusion appears to apply to this subgroup of patients, clinical evidence for this approach is lacking. An aspect that is particularly relevant for the Asian-Pacific situation is the use of traditional herbal medication, which may interact with antiplatelets and anticoagulants [43].
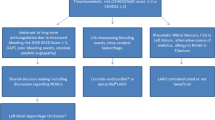
A decision tree, based on the EHRA/EAPCI consensus statement on LAAO [23] and adapted for the Asian-Pacific setting, is shown in Fig. 1.
Decision tree regarding available treatments for patients with NVAF at risk of ischemic stroke accounting for specific aspects applicable to Asian-Pacific patients (adapted from EHRA/EAPCI consensus statement on LAAO [23])
2.4 How to ensure procedural success of LAAO?
Despite the advancements in LAA occluders, ancillary equipment, and clinical experience, LAAO remains a technically challenging procedure. Availability of and experience with modern imaging techniques and a skilled implantation team trained and proctored by experienced operators may be crucial factors for achieving procedural success of LAAO. Transesophageal echocardiography (TEE) is the most frequently used imaging modality for LAAO implantation procedures, although it requires the procedure to be performed under general anesthesia. Intracardiac echocardiography (ICE) does not require an additional operator or general anesthesia, but does not provide the broad view of TEE and involves higher equipment costs. Skillfully operated imaging is essential for identifying pericardial effusion and tamponade, which are typical procedural complications of LAAO [44]. The implantation team should be well prepared to resolve this complication (e.g., by having a pericardial drainage kit readily available during the procedure), as well as to perform percutaneous retrieval of an embolized occluder. In addition, cardiac surgery should be available if these complications cannot be resolved by less invasive methods. Consensus with respect to achieving procedural success of LAAO is summarized in Table 5.
2.5 Concomitant procedures
Combination of implantation of an LAAO device with other interventional procedures has been reported by several authors. Reported concomitant procedures include catheter ablation for atrial fibrillation [45], MitraClip implantation [46], closure of a patent foramen ovale or atrial septal defect [47], and transcatheter aortic valve implantation, in some cases also combined with percutaneous coronary intervention [48]. These reports suggest the feasibility and acceptable safety of concomitant procedures during LAAO implantation, although with a higher exposure to radiation and contrast and involving longer procedural duration. However, the number of reported cases is relatively low and insufficient to provide convincing clinical evidence. Overall, concomitant procedures should be justified by a rationale accounting for procedural/technical aspects and patient safety. Especially if an additional access route is required besides the venous-transseptal access used for LAAO implantation, the feasibility and overall reduction of procedural risks should be critically assessed. Moreover, concomitant procedures may result in an increased use of contrast medium and therefore require careful monitoring of renal function. Besides the clinical benefit-risk considerations, cultural, economic, and logistic aspects may play a specific role in the Asian-Pacific context, for instance, the fact that patients may reside more remote from interventional cardiology centers than in the European and US situation and may prefer some procedures to be done together. Consensus regarding concomitant procedures is summarized in Table 6.
2.6 Postprocedural treatment and TEE concept
The EHRA/EAPCI consensus statements on LAAO recommend dual APT for at least 1 month after device implantation for LAAO or until follow-up by TEE has been performed. After this initial period, long-term single APT is recommended. The primary objective of performing postprocedural follow-up using TEE is to identify peri-device leak and DRT. While the clinical significance of residual leak is not clearly determined, DRT is considered a reason to initiate an intensified antithrombotic regime until the thrombus has been resolved [49]. Such an intensified therapy should account for the patient’s tolerance to antithrombotic medication and device-specific recommendations and may typically include intravenous heparin or NOACs. The timing of subsequent TEE follow-up should be aligned with the antithrombotic regime, i.e., a change or discontinuation of the therapy should be confirmed by the outcome of TEE. The mechanism of DRT formation still remains relatively unclear, including implantation and patient conditions and device aspects promoting DRT as well as its timing and natural history. An overview of the consensus on postprocedural treatment and TEE imaging is provided in Table 7.
3 Discussion
For patients with NVAF at risk for ischemic stroke, oral anticoagulation therapy is the mainstream evidence-based treatment for ischemic stroke prevention. However, based on absolute contraindications or elevated bleeding risk, LAAO is a feasible alternative for carefully selected NVAF patients. Clinical evidence for LAAO has been provided by randomized controlled trials and large prospective registries, although most of the data was obtained from Caucasian patients. Asian-Pacific patients have specific stroke and bleeding risks, which may influence the benefit-risk profile of LAAO for these patients. This requires an individualized approach to LAAO in Asian-Pacific patients, accounting for their specific risk profile and aspects typical for the Asian-Pacific healthcare context. This current consensus statement was developed to provide guidance in this clinical decision-making process. During the process of developing this consensus, a number of uncertainties and unanswered questions were identified, applicable to any NVAF patients at risk for ischemic stroke or to Asian-Pacific patients more specifically including:
-
Ischemic stroke and bleeding risks in Asian-Pacific patients and effect of these risks on eligibility for LAAO versus medical treatment. Areas of particular interest include the risks and benefits of LAAO in Asian-Pacific patients compared with those receiving NOAC or APT therapy (including dosing per label and off-label underdosing) and LAAO in specific subgroups of Asian-Pacific patients.
-
Safety and effectiveness of LAAO in specific patient groups, with evidence specifically lacking on patients with electrically isolated LAA after ablation and patients not eligible for any antithrombotic regiment after LAAO implantation.
-
Benefit-risk profile of procedures combining LAAO implantation with other interventions, accounting for specific Asian-Pacific aspects, such as access to healthcare and available treatment alternatives.
-
Safety and feasibility of TAVI as an intervention concomitant to LAAO implantation.
-
Mechanism and clinical significance of DRT formation and consequences for post-implantation TEE follow-up.
There is a need for additional clinical data to answer these questions. Prospective registries, preferably including a comparison with patients receiving medical treatment, appear to be the most appropriate and ethical method to obtain these data. Currently, there is no data from prospective registries in Asian populations regarding the above aspects specifically or on LAAO in general.
Implantation of an LAAO device is a technically challenging procedure. Prerequisites for a safe and successful procedure include a highly skilled implantation team, knowledge of procedural complications and their resolution, and state-of-the-art equipment. In addition, pre-implant imaging to assess the relevant anatomy, exclude LAA and left atrial thrombus, and determine the size of the LAAO is instrumental to procedural success. Following implantation, TEE imaging is the preferred imaging modality to identify device-related complications, including device embolization, DRT, and peri-device leaks. The LAAO implantation team, including the echocardiography operator, should be thoroughly trained on these aspects, preferably with the involvement of an experienced proctoring physician.
The expert opinions and consensus presented here are based on current knowledge and insights. LAAO is currently in an evolving stage in many Asian-Pacific countries, and opinions and consensus may need future updates when more clinical data becomes available from this region.
References
Ezekowitz MD, Bridgers SL, James KE, Carliner NH, Colling CL, Gornick CC, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans affairs stroke prevention in nonrheumatic atrial fibrillation investigators. N Engl J Med. 1992;327:1406–12.
Singer DE, Hughes RA, Gress DR, Sheehan MA, Oertel LB, Maraventano SW, et al. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505–11.
The stroke prevention in atrial fibrillation investigators. Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527–39.
Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–96.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104.
Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–9.
Cresti A, García-Fernández MA, Sievert H, Mazzone P, Baratta P, Solari M, et al. Prevalence of extra-appendage thrombosis in non-valvular atrial fibrillation and atrial flutter in patients undergoing cardioversion: a large transoesophageal echo study. EuroIntervention. 2019;15:e225–30.
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–42.
Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12.
Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER cardiac plug. EuroIntervention. 2016;11:1170–9.
Landmesser U, Tondo C, Camm J, Diener HC, Paul V, Schmidt B, et al. Left atrial appendage occlusion with the AMPLATZER amulet device: one-year follow-up from the prospective global amulet observational registry. EuroIntervention. 2018;14:e590–e7.
Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–8.
Holmes DR, Reddy VY, Buchbinder M, Stein K, Elletson M, Bergmann MW, et al. The assessment of the Watchman device in patients unsuitable for oral anticoagulation (ASAP-TOO) trial. Am Heart J. 2017;189:68–74.
Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011–21.
Hausler KG, Endres M, Landmesser U. Left atrial appendage occlusion in patients with nonvalvular atrial fibrillation : present evidence, ongoing studies, open questions. Med Klin-Intensivmed. 2018. https://doi.org/10.1007/s00063-018-0500-4.
Tilz RR, Potpara T, Chen J, Dobreanu D, Larsen TB, Haugaa KH, et al. Left atrial appendage occluder implantation in Europe: indications and anticoagulation post-implantation. Results of the European Heart Rhythm Association Survey. Europace. 2017;19:1737–42.
Schmidt B, Chun KR. Antithrombotic therapy after left atrial appendage closure. Expert Rev Cardiovasc Ther. 2015;13:105–9.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–32.
Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. EuroIntervention. 2020;15:1133–80.
Casu G, Gulizia MM, Molon G, Mazzone P, Audo A, Casolo G, et al. ANMCO/AIAC/SICI-GISE/SIC/SICCH consensus document: percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation patients: indications, patient selection, staff skills, organisation, and training. Eur Heart J Suppl. 2017;19:D333–D53.
Kavinsky CJ, Kusumoto FM, Bavry AA, Bailey SR, Ellenbogen KA, Hess PL, et al. SCAI/ACC/HRS institutional and operator requirements for left atrial appendage occlusion. J Am Coll Cardiol. 2016;67:2295–305.
Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GYH. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147:109–19.
Phillips KP, Santoso T, Sanders P, Alison J, Chan JLK, Pak H-N, et al. Left atrial appendage closure with WATCHMAN in Asian patients: 2 year outcomes from the WASP registry. Int J Cardiol Heart Vasc. 2019;23:100358.
Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–97.
Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891–6.
Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–54.
Chan YH, Lee HF, See LC, Tu HT, Chao TF, Yeh YH, et al. Effectiveness and safety of four direct oral anticoagulants in Asian patients with nonvalvular atrial fibrillation. Chest. 2019;156:529–43.
Zhang J, Tang J, Cui X, Wang B, Bu M, Bai Y, et al. Indirect comparison of novel oral anticoagulants among Asians with non-Valvular atrial fibrillation in the real world setting: a network meta-analysis. BMC Cardiovas Disord. 2019;19:182.
Lee SR, Lee YS, Park JS, Cha MJ, Kim TH, Park J, et al. Label adherence for non-vitamin K antagonist oral anticoagulants in a prospective cohort of Asian patients with atrial fibrillation. Yonsei Med J. 2019;60:277–84.
Yasaka M, Lip GY. Impact of non-vitamin K antagonist oral anticoagulants on intracranial bleeding in Asian patients with non-valvular atrial fibrillation. Circ J. 2014;78:2367–72.
Shen AY-J, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–15.
Bang OY, Hong K-S, Heo JH. Asian patients with stroke plus atrial fibrillation and the dose of non-vitamin K oral anticoagulants. J Stroke. 2016;18:169–78.
Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener H-C, Dubner SJ, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol. 2017;69:777–85.
Chan YH, Kuo CT, Yeh YH, Chang SH, Wu LS, Lee HF, et al. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;68:1389–401.
Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017;48:3040–8.
Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol. 2016;68:2597–604.
Chao T-F, Lip GYH, Liu C-J, Tuan T-C, Chen S-J, Wang K-L, et al. Validation of a modified CHA2DS2-VASc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47:2462–9.
DiBiase L, Mohanty S, Trivedi C, Romero J, Natale V, Briceno D, et al. Stroke risk in patients with atrial fibrillation undergoing electrical isolation of the left atrial appendage. J Am Coll Cardiol. 2019;74:1019–28.
Tsai HH, Lin HW, Lu YH, Chen YL, Mahady GB. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS One. 2013;8:e64255.
Price MJ. Prevention and management of complications of left atrial appendage closure devices. Interv Cardiol Clin. 2014;3:301–11.
Du X, Chu H, Ye P, He B, Xu H, Jiang S, et al. Combination of left atrial appendage closure and catheter ablation in a single procedure for patients with atrial fibrillation: multicenter experience. J Formos Med Assoc. 2019;118:891–7.
Kuwata S, Taramasso M, Zuber M, Suetsch G, Attinger-Toller A, Wicki D, et al. Feasibility of concomitant MitraClip and left atrial appendage occlusion. EuroIntervention. 2017;12:1940–5.
Yu J, Liu X, Zhou J, Xue X, Muenzel M, Schulze PC, et al. Long-term safety and efficacy of combined percutaneous LAA and PFO/ASD closure: a single-center experience (LAAC combined PFO/ASD closure). Expert Rev Med Devices. 2019;16:429–35.
Khattab AA, Gloekler S, Sprecher B, Shakir S, Guerios E, Stortecky S, et al. Feasibility and outcomes of combined transcatheter aortic valve replacement with other structural heart interventions in a single session: a matched cohort study. Open Heart. 2014;1:e000014.
Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528–36.
Acknowledgments
This expert opinion and consensus statement is based on discussions among clinical experts listed below during the Heart & Brain Summit in Bangkok, Thailand, July 13 and 14, 2019. All experts listed below reviewed and agreed with the final consensus manuscript.
T. Akagi, Okayama, Japan; J.L.K. Chan, Hong Kong; C.T. Chi, Vietnam; C-C. Chiu, Chang Hua, Taiwan; A.S.F. Chui, Hong Kong; K. Durongpisitkul, Bangkok, Thailand; E. Harmeiwaty, Jakarta, Indonesia; D. Firman, Jakarta, Indonesia; S. Haifeng, Beijing, China; P.K. Hazra, Kolkata, India; S. Komonchan, Bangkok, Thailand; P.X. Lanh, Ho Chi Minh City, Vietnam; M-C. Lin, Taichung, Taiwan; H.F. Liong, Kuala Lumpur, Malaysia; K. Meemook, Bangkok, Thailand; A.M. Muthuppalaniappan, Kuala Lumpur, Malaysia; N.H.T. My, Ho Chi Minh City, Vietnam; Y. Nakajima, Iwate, Japan; K. Oki, Tokyo, Japan; B. Paul, Kolkata, India; A.T. Prabhakar, Vellore, India; P. Puhl, Sydney, Australia; S. Pumprueg, Bangkok, Thailand; D.A. Quoc, Ho Chi Minh City, Vietnam; V. Samuel, Vellore, India; L. Sanders, Melbourne, Australia; V. Sharma, Singapore; J. Sharp, Sydney, Australia; R. Sharpe, Gold Coast, Australia; E. Tay, Singapore; N.B. Thang, Ho Chi Minh City, Vietnam; D. Wang, Sydney, Australia; A. Wong, Brisbane, Australia; X. Yingqi, Jilin, China; W. Zhanhang, Guangzhou, China.
Funding
Organization of the Heart & Brain Summit in Bangkok, Thailand, July 13 and 14, 2019, was logistically and financially supported by Abbott.
Author information
Authors and Affiliations
Contributions
Development of this consensus was initiated following several indications from Asian-Pacific physicians regarding the paucity of clinical evidence for LAAO obtained from Asian-Pacific patients. The consensus presented in this manuscript is the result of discussions during a 2-day consensus meeting in which all contributors listed above participated and provided input to the arrived consensus. These participants were selected based on their expertise and experience in ischemic stroke prevention and LAAO. This manuscript was prepared by Dr. Albers in close collaboration with Dr. Diener and Dr. Lewalter and was reviewed by all authors and by all participants in the consensus meeting.
Corresponding author
Ethics declarations
Conflict of interest
Edgar Tay is a speaker and proctor and received an educational grant from Boston Scientific. Jason Sharp is a proctor for Abbott and Boston Scientific. Dennis Wang is a proctor for Abbott. Angus Shing Fung Chui is a proctor for Boston Scientific. Bert Albers received a honorarium from Abbott for preparing this manuscript. In the last 3 years, Hans-Christoph Diener (HCD) received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from Abbott, Bayer Vital, BMS, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Pfizer, Portola, Sanofi-Aventis, and WebMD Global. Financial support for research projects was provided by Boehringer Ingelheim. HCD received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. HCD served as editor of Neurologie up2date, Info Neurologie & Psychiatrie, and Arzneimitteltherapie and is on the editorial board of Lancet Neurology. HCD chairs the Treatment Guidelines Committee of the German Society of Neurology and contributed to the EHRA and ESC guidelines for the treatment of AF. Thorsten Lewalter reported lectures and memberships of advisory boards with modest honoraria for Abbott, Boston Scientific, Lifetech, Bayer, Daiichi Sankyo, Pfizer, and Böhringer Ingelheim. Andrew Wong reports no disclosures or conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Societal endorsement: This paper is endorsed by the Asian Pacific Society of Interventional Cardiology (APSIC).
Rights and permissions
About this article
Cite this article
Tay, E., Paul, B., Sharp, J. et al. Left atrial appendage occlusion for ischemic stroke prevention in patients with non-valvular atrial fibrillation: clinical expert opinion and consensus statement for the Asian-Pacific region. J Interv Card Electrophysiol 61, 269–281 (2021). https://doi.org/10.1007/s10840-020-00752-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00752-8