Abstract
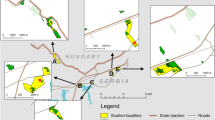
Mobility is crucial for the maintenance of viable metapopulations, but quantitative data to evaluate risks due to insufficient individual mobility of focal insect species are mostly lacking. We selected the butterfly Brenthis ino, a species typically confined to wet fallow grasslands in Central Europe and performed a mark–release–recapture study in a 3.2 ha study area with one big and one small patch of suitable habitat from 22 June to 23 July 2010. The position of each butterfly capture was measured with a GPS and transferred into a GIS. In total, we marked 984 individuals in 1,545 capture events and estimated that the cumulative population size was 2,400 individuals. The initial increase of adult males proceeded much faster than for females, similar to the protandrous population build-up known from other butterflies. Moved distances for both sexes usually did not exceed 80 m, and about 40 % of all individuals used less than 2 % of the available suitable habitat. All individuals switching to the other patch returned later to their patch of origin, confirming that B. ino is highly philopatric. We conclude that low effective mobility in B. ino produces much smaller home ranges than suggested by merely observing flight activities in the field, and that low tendencies towards long-distance movements significantly hamper the maintenance of metapopulations when patch density decreases due to landscape fragmentation.






Similar content being viewed by others
References
Baguette M (2003) Long distance dispersal and landscape occupancy in a metapopulation of the cranberry fritillary butterfly. Ecography 26:153–160
Baguette M, van Dyck H (2007) landscape connectivity and animal behaviour: functional grain as key determinant for dispersal. Landscape Ecol 22:1117–1129
Baguette M, Petit S, Quéva F (2000) Population spatial structure and migration of three butterfly species within the same habitat network: consequences for conservation. J Appl Ecol 37:100–108
Baker RR (1968) Sun orientation during migration in some British butterflies. Proc R Entomol Soc Lond A 43:89–95
Baker RR (1969) The evolution of the migratory habit in butterflies. J Anim Ecol 38:703–746
Baker RR (1983) Insect territoriality. Ann Rev Entomol 28:65–89
Baker RR (1984) The dilemma: when and how to go or stay. In: Vane-Wright RI, Ackery PR (eds) The biology of butterflies, vol 11. Academic Press, London, pp 279–296
Barton BJ, Bach CE (2005) Habitat Use by the federally endangered Mitchell’s Satyr Butterfly (Neonympha mitchellii mitchellii) in a Michigan Prairie Fen. Am Midl Nat 153:41–51
Bélisle M (2005) Measuring landscape connectivity: the challenge of behavioral landscape ecology. Ecology 86:1988–1995
Bennet AF (1999) Linkages in the landscape: the role of corridors and connectivity in wildlife conservation. IUCN Publications, Cambridge
Bitzer RJ, Shaw KC (1995) Territorial behaviour of the red admiral, Vanessa atalanta (Lepidoptera: Nymphalidae). I. The role of climatic factors and early interaction frequency on territorial start time. J Insect Behav 8:47–66
Brinson MM, Malvárez AI (2002) Temperate freshwater wetlands: types, status, and threats. Environ Conserv 29:115–133
Burgman MA, Fox JC (2003) Bias in species range estimates from minimum convex polygons: implications for conservation and options for improved planning. Anim Conserv 6:19–28
Caro T (1999) The behaviour-conservation interface. Trends Ecol Evol 14:490
Conradt L, Bodsworth EJ, Roper TJ, Thomas CD (2000) Non-random dispersal in the butterfly Maniola jurtina: implications for metapopulation models. Proc R Entomol Soc Lond B 267:1505–1510
Conradt L, Roper TJ, Thomas CD (2001) Dispersal behaviour of individuals in metapopulations of two British butterflies. Oikos 95:416–424
Cooch E, White GC (2007) Program MARK. A gentle introduction, 6th edn. http://www.phidort.org/software/mark/docs/book. Accessed on 2 Sept 2010
Cooper CB, Walters JR (2002) Experimental evidence of disrupted dispersal causing decline of an Australian passerine in fragmented habitat. Conserv Biol 16:471–478
Cozzi G, Müller CB, Krauss J (2008) How do local habitat management and landscape structure at different spatial scales affect fritillary butterfly distribution in fragmented wetlands? Landscape Ecol 23:269–283
Dover JW (1991) The Conservation of insects on arable farmland. In: Collins NM, Thomas JA (eds) The conservation of insects and their habitats. Academic Press, London, pp 294–318
Dover JW, Rowlingson B (2005) The western jewel butterfly (Hypochrysops halyaetus): factors affecting adult butterfly distribution within native Banksia bushland in an urban setting. Biol Conserv 122:599–609
Ebert G, Rennwald E (eds) (1991) Die Schmetterlinge Baden-Württembergs Band 1 Tagfalter 1, Ulmer, pp: 445ff–451, 552 pp
ESRI (1996) ArcView GIS 3.2 reference manual for window by ESRI. Environment Systems and Research Institute, New York Street, Redlands CA
Etheredge JA, Perez SM, Taylor OR, Jander R (1999) Monarch butterflies (Danaus plexippus L.) use a magnetic compass for navigation. PNAS 96:13845–13846
Fischer K, Fiedler K (2001) Resource-based territoriality in the butterfly Lycaena hippothoe and environmentally induced behavioural shifts. Anim Behav 61:723–732
Fric Z, Konvicka M (2007) Dispersal kernels of butterflies: power-law functions are invariant to marking frequency. Basic Appl Ecol 8:377–386
Fric Z, Hula V, Klimova M, Zimmermann K, Konvicka M (2009) Dispersal of four fritillary butterflies within identical landscape. Ecol Res 25:543–552
Gibbs JP (2000) Wetland loss and biodiversity conservation. Conserv Biol 14:314–317
Haddad NM (1999) Corridor use predicted from behaviour at habitat boundaries. Am Nat 153:215–227
Haddad NM, Bowne DR, Cunninghamm A, Danielson BJ, Levey DJ, Sargent S, Spira T (2003) Corridor use by diverse taxa. Ecology 84:609–615
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I, Ovaskainen O (2003) Metapopulation theory for fragmented landscapes. Theor Popul Biol 64:119–127
Hanski I, Stevnes PC, Ihalempiä P, Selonen V (2000) Home-range size, movements, and nest-site use in the Siberian flying squirrel, Pteromys volans. J Mammal 81:798–809
Hartig EK, Grozev O, Rosenzweig C (1997) Climate change, agricultural and wetlands in Eastern Europe: vulnerability, adaptation and policy. Clim Change 36:107–121
Hill JK, Thomas CD, Lewis OT (1996) Effects of habitat patch size and isolation on dispersal by Hesperia comma butterflies: implications for metapopulation structure. J Anim Ecol 65:725–735
Hölldobler B (1976) Recruitment behaviour, home range orientation and territoriality in harvester ants Pogonomyrmex. Behav Ecol Sociobiol 1:3–44
Hovestadt T, Nowicki P (2008) Investigating movement within irregularly shaped patches: analysis of mark–release–recapture data using randomisation procedures. Israel J Ecol Evol 54:137–154
Hovestadt T, Nowicki P, Binzenhöfer B, Settele J (2011) Do all inter-patch movements represent dispersal? A mixed kernel study of butterfly mobility in fragmented landscapes. J Anim Ecol 80:1070–1077
Junker M, Schmitt T (2010) Demography, dispersal and movement pattern of Euphydryas aurina (Lepidoptera: Nymphalidae) at the Iberian Peninsula: an alarming example in an increasingly fragmented landscape? Insect Conserv 14:237–246
Junker M, Wagner S, Gros P, Schmitt T (2010) Changing demography and dispersal behaviour: ecological adaptation in an alpine butterfly. Oecologia 164:971–980
Kudrna O, Harpke A, Lux K, Pennersdorfer J, Schweiger O, Settele J, Wiemers M (2011) Distribution atlas of butterflies in Europe. Gesellschaft für Schmetterlingsschutz, Halle
Kuras T, Benes J, Fric Z, Konvicka M (2003) Dispersal patterns of endemic alpine butterflies with contrasting population structures: Erebia epiphron and E. sudetica. Popul Ecol 45:115–123
Lidicker WZ Jr (1975) The role of dispersal in the demography of small mammals. In: Golley FB, Perturusewicz K, Ryszkowski L (eds) Small mammals: their productivity and population dynamics. Cambridge University Press, London, pp 103–108
Lurtz PWW, Garson PJ, Wauters LA (1997) Effects of temporal and spatial variation in habitat quality on red squirrel dispersal behaviour. Anim Behav 54:427–435
Mallet J (1986) Dispersal and gene flow in a butterfly with home range behaviour: Heliconius erato (Lepidoptera: Nymphalidae). Oecologia 68:210–217
Mittelstaedt H (1962) Control systems of orientation in insects. A Rev Entomol 7:177–198
Moore D, van Nest BN, Seier E (2011) Diminishing returns: the influence of experience and environment on time-memory extinction in honeybee foragers. J Comp Physiol A. doi:10.1007/s00359-011-0624-y
Munguia ML, Martin J, Gracia-Barros E, Viejo JL (1997) Use of space and resources in a Mediterranean population of the butterfly Euphydryas aurinia. Acta Oecol 17:597–612
Öckinger E, Hammerstedt O, Nilsson SG, Smith HG (2006) The relationship between local extinctions of grassland butterflies and increased soil nitrogen levels. Biol Conserv 128:564–573
Parmesan C (1996) Climate and specie’s range. Nature 382:765–766
Poethke HJ, Hovestadt T (2002) Evolution of density and patch size dependent dispersal rates. Proc R Soc Lond B 269:637–645
R development core team (2009) R: a language and environment for statistical computing. Vienna, Austria. http://www.R-project.org. Accessed on 13 Nov 2010
Reed JM (1999) The role of behaviour in recent avian extinctions and endangerments. Conserv Biol 13:232–241
Root RB, Kareiva PM (1984) The search for resources by cabbage butterflies (Pieris rapae): ecological consequences and adaptive significance of Markovian movements in a patchy environment. Ecology 65:147–165
Schneider C, Dover J, Fry GLA (2003) Movement of two grassland butterflies in the same habitat network: the role of adult resources and size of the study area. Ecol Entomol 28:219–227
Schtickzelle N, Baguette M (2003) Behavioural responses to habitat patch boundaries restricts dispersal and generates emigration-patch area relationships in fragmented landscapes. J Anim Ecol 72:533–545
Settele J, Feldmann R, Reinhardt R (1999a) Die Tagfalter Deutschlands—Ein Handbuch für Freilandökologen, Umweltplaner und Naturschützer. Ulmer, Stuttgart, p 452
Settele J, Feldmann R, Henle K, Kockelke K, Poethke HJ (1999b) Methoden der quantitativen Erfassung von Tagfaltern. In: Settele J, Feldmann R, Reinhardt R (eds) Die Tagfalter Deutschlands. Ulmer, Stuttgart, pp 144–186, p 452
Shier DM (2006) Effect of family support on the success of translocated black-tailed prairie dogs. Conserv Biol 20:1780–1790
Shreeve TG, Dennis RLH (2011) Landscape scale conservation: resources, behaviour, the matrix and opportunities. J Insect Conserv 15:179–188
Stevens VM, Turlure C, Baguette M (2010) A meta-analysis of dispersal in butterflies. Biol Rev 85:625–642
Strier KB (1997) Behavioural ecology and conservation biology of primates and other animals. Adv Stud Behav 26:101–158
Sutherland WJ (1998) The importance of behavioural studies in conservation biology. Anim Behav 56:801–809
Sutherland WJ, Dolman PM (1994) Combining behaviour and population dynamics with applications for predicting consequences of habitat loss. Proc R Soc Lond B 255:955–963
Tolman T, Levington R (1998) Die Tagfalter Europas und Nordwestafrikas. Kosmos Verlags-GmbH & Co-Stuttgart, 319 pp
Trakhtenbrot A, Nathan R, Perry G, Richardson DM (2005) The importance of long-distance dispersal in biodiversity conservation. Divers Distrib 11:173–181
Turner JRG (1971) Experiments on the demography of tropical butterflies. II. Longevity and home-range behaviour in Heliconius. Biotropica 3:21–31
van Dyck H, Baguette M (2005) Dispersal behaviour in fragmented landscapes: routine or special movements? Basic Appl Ecol 6:535–545
Van Swaay C, Cuttelod A, Collins S, Maes D, Lopez M, Munguira M, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhof I (2010) European red list of butterflies. Publications Office of the European Union, Luxembourg
Wahlberg N, Klemetti T, Hanski I (2002) Dynamic populations in a dynamic landscape: the metapopulation structure of the marsh fritillary butterfly. Ecography 25:224–232
Wolff JO, Lidicker WZ Jr (1980) Population ecology of the taiga vole, Microtus xanthognathus, in interior Alaska. Can Zool 58:1800–1812
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Zimmermann K, Fric Z, Filipová L, Konvicka M (2005) Adult demography, dispersal and behaviour of Brenthis ino (Lepidoptera: Nymphalidae): how to be a successful wetland butterfly. Eur J Entomol 102:699–706
Acknowledgments
We acknowledge the “Forschungsinitiative Rheinland-Pfalz” for financing the employment of Jessica Weyer and the DFG graduate school “Verbesserung von Normsetzung und Normanwendung im integrierten Umweltschutz durch rechts- und naturwissenschaftliche Kooperation” (No. 1319) Trier University for scientific support. We are grateful to Dr. Ortwin Elle for advice and support in the GIS analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weyer, J., Schmitt, T. Knowing the way home: strong philopatry of a highly mobile insect species, Brenthis ino . J Insect Conserv 17, 1197–1208 (2013). https://doi.org/10.1007/s10841-013-9601-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-013-9601-9