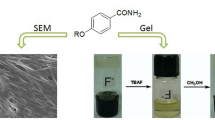
Abstract
We reported a supramolecular system consisted of β-cyclodextrin, N,N-dimethylformamide and LiCl, which could exhibit different behaviors toward various alcohols. When some liquid monohydric alcohols were injected into the system at room temperature, a semitransparent organogel (the ambient temperature organogel) was formed. Compared with liquid monohydric alcohols, the addition of solid alcohols could induce the formation of a heat-set organogel, a solution, and an ice-like crystal at different temperatures. The xerogels and dried ice-like crystal were characterized by scanning electron microscope, Fourier transform infrared spectroscopy, X-ray powder diffraction, thermogravimetry and derivative thermogravimetry. The systems were also studied by 1H nuclear magnetic resonance and 2D rotating frame overhauser effect spectroscopy. The alcohol-responsive properties of this system could be further designed as molecule switches based on molecular recognition.
Graphical Abstract
The mechanism of the three-dimensional network formation by self-assembly in the systems: a, LiCl and heat (or liquid monohydric alcohol); b, LiCl, solid alcohol and heat (or cool). We found a novel supramolecular system containing β-cyclodextrin. It was a clear solution at room temperature and could form a heat-set organogel by heating. It could exhibit different behaviors toward various alcohols: ambient temperature organogel, ice-like crystal, heat-set organogel or solution.











Similar content being viewed by others
References
Ardá, A., Blasco, P.D., Silva, V., Schubert, V., André, S., Bruix, M., Cañada, F.J., Gabius, H.J., Unverzagt, C., Jiménez-Barbero, J.: Molecular recognition of complex-type biantennary N-glycans by protein receptors: a three-dimensional view on epitope selection by NMR. J. Am. Chem. Soc. 135, 2667–2675 (2013)
Ma, M., Paredes, A., Bong, D.: Intra- and inter-membrane pairwise molecular recognition between synthetic hydrogen-bonding phospholipids. J. Am. Chem. Soc. 130, 14456–14458 (2008)
Oleksy, A., Blanco, A.G., Boer, R., Usón, I., Aymamí, J., Rodger, A., Hannon, M.J., Coll, M.: Molecular recognition of a three-way DNA junction by a metallosupramolecular helicate. Angew. Chem. Int. Ed. 45, 1227–1231 (2006)
Zhang, H., Li, F., Dever, B., Li, X.F., Le, X.C.: DNA-mediated homogeneous binding assays for nucleic acids and proteins. Chem. Rev. 113, 2812–2841 (2013)
Hargrove, A.E., Nieto, S., Zhang, T., Sessler, J.L., Anslyn, E.V.: Artificial receptors for the recognition of phosphorylated molecules. Chem. Rev. 111, 6603–6782 (2011)
Mahadevi, A.S., Sastry, G.N.: Cation–π interaction: its role and relevance in chemistry, biology, and material science. Chem. Rev. 113, 2100–2138 (2013)
Hermann, T., Patel, D.J.: Adaptive recognition by nucleic acid aptamers. Science 287, 820–825 (2000)
Dong, S., Luo, Y., Yan, X., Zheng, B., Ding, X., Yu, Y., Ma, Z., Zhao, Q., Huang, F.: A dual-responsive supramolecular polymer gel formed by crown ether based molecular recognition. Angew. Chem. 50, 1905–1909 (2011)
Wei, B., Mi, Y.: Aptamer based reversible DNA induced hydrogel system for molecular recognition and separation. Chem. Commun. 46, 6308–6310 (2010)
Danjo, H., Hirata, K., Yoshigai, S., Azumaya, I., Kentaro, Y.: Back to back twin bowls of D 3-symmetric tris (spiroborate) s for supramolecular chain structures. J. Am. Chem. Soc. 131, 1638–1639 (2009)
Maeda, K., Mochizuki, H., Osato, K., Yashima, E.: Stimuli-responsive helical poly(phenylacetylene)s bearing cyclodextrin pendants that exhibit enantioselective gelation in response to chirality of a chiral amine and hierarchical super-structured helix formation. Macromolecules 44, 3217–3226 (2011)
Harada, A., Kobayashi, R., Takashima, Y., Hashidzume, A., Yamaguchi, H.: Macroscopic self-assembly through molecular recognition. Nat Chem 3, 34–37 (2010)
Yamaguchi, H., Kobayashi, R., Takashima, Y., Hashidzume, A., Harada, A.: Self-assembly of gels through molecular recognition of cyclodextrins: shape selectivity for linear and cyclic guest molecules. Macromolecules 44, 2395–2399 (2011)
Li, Y., Liu, J., Du, G., Yan, H., Wang, H., Zhang, H., An, W., Zhao, W., Sun, T., Xin, F., Kong, L., Li, Y., Hao, A., Hao, J.: Reversible heat-set organogel based on supramolecular interactions of β-cyclodextrin in N,N-dimethylformamide. J. Phys. Chem. B 114, 10321–10326 (2010)
Liu, W., Xing, P., Xin, F., Hou, Y., Sun, T., Hao, J., Hao, A.: Novel double phase transforming organogel based on beta-cyclodextrin in 1,2-propylene glycol. J. Phys. Chem. B 116, 13106–13113 (2012)
Xin, F., Zhang, H., Hao, B., Sun, T., Kong, L., Li, Y., Hou, Y., Li, S., Zhang, Y., Hao, A.: Controllable transformation from sensitive and reversible heat-set organogel to stable gel induced by sodium acetate. Colloids Surf. A 410, 18–22 (2012)
Hou, Y., Xin, F., Yin, M., Kong, L., Zhang, H., Sun, T., Xing, P., Hao, A.: Stimuli-responsive supramolecular organogels that exhibit a succession of micro-morphologies. Colloids Surf. A 414, 160–167 (2012)
Zhu, P., Yan, X., Su, Y., Yang, Y., Li, J.: Solvent-induced structural transition of self-assembled dipeptide: from organogels to microcrystals. Chem. Eur. J. 16, 3176–3183 (2010)
Kida, T., Marui, Y., Miyawaki, K., Kato, E., Akashi, M.: Unique organogel formation with a channel-type cyclodextrin assembly. Chem. Commun. 16, 3889–3891 (2009)
Guo, X.Q., Song, L.X., Du, F.Y., Dang, Z., Wang, M.: Important effects of lithium carbonate on stoichiometry and property of the inclusion complexes of polypropylene glycol and β-cyclodextrin. J. Phys. Chem. B 115, 1139–1144 (2011)
Bolla, G., Nangia, A.: Clofazimine mesylate: a high solubility stable salt. Cryst. Growth Des. 12, 6250–6259 (2012)
Ohta, N., Fuyuhiro, A., Yamanari, K.: Complete enantioseparation through supramolecular complex formation between tris (1,3-diaminopropane) cobalt (III) phosphate and β-cyclodextrin,[Co (tn) 3] PO4·β-CDX. Inorg. Chem. 49, 9122–9124 (2010)
Liu, B., Zhao, J., Liu, Y., Zhu, X., Zeng, J.: Physiochemical properties of the inclusion complex of puerarin and glucosyl-β-cyclodextrin. J. Agric. Food Chem. 60, 12501–12507 (2012)
Miura, T., Kida, T., Akashi, M.: Recognition of stereoregularity of poly (methacrylic acid) s with γ-cyclodextrin. Macromolecules 44, 3723–3729 (2011)
Ng, E.P., Chateigner, D., Bein, T., Valtchev, V., Mintova, S.: Capturing ultrasmall EMT zeolite from template-free systems. Science 335, 70–73 (2012)
Chung, J.W., Kwak, S.Y.: Iron-induced cyclodextrin self-assembly into size-controllable nanospheres. Langmuir 26, 2418–2423 (2009)
Song, L.X., Pan, S.Z., Zhu, L.H., Wang, M., Du, F.Y., Chen, J.: Molecule-ion interaction and its effect on coordination interaction. Inorg. Chem. 50, 2215–2223 (2011)
Liu, Y., Zhao, Y.L., Chen, Y., Wang, M.: Supramolecular assembly of gold nanoparticles mediated by polypseudorotaxane with thiolated β-cyclodextrin. Macromol. Rapid Commun. 26, 401–406 (2005)
Gao, Y.A., Li, Z.H., Du, J.M., Han, B.X., Li, G.Z., Hou, W.G., Shen, D., Zheng, L.Q., Zhang, G.Y.: Preparation and characterization of inclusion complexes of β-cyclodextrin with ionic liquid. Chem. Eur. J. 11, 5875–5880 (2005)
Song, L.X., Yang, J., Bai, L., Du, F.Y., Chen, J., Wang, M.: Molecule-ion interaction and its effect on electrostatic interaction in the system of copper chloride and β-cyclodextrin. Inorg. Chem. 50, 1682–1688 (2011)
Song, L.X., Du, F.Y., Guo, X.Q., Pan, S.Z.: Formation, characterization, and thermal degradation behavior of a novel tricomponent aggregate of β-cyclodextrin, ferrocene, and polypropylene glycol. J. Phys. Chem. B 114, 1738–1744 (2010)
Uekama, K., Fujinaga, T., Hirayama, F., Otagiri, M., Yamasaki, M., Seo, H., Hashimoto, T., Tsuruoka, M.: Improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation. J. Pharm. Sci. 72, 1338–1341 (1983)
Franco, C., Schwingel, L., Lula, I., Sinisterra, R.D., Koester, L.S., Bassani, V.L.: Studies on coumestrol/β-cyclodextrin association: inclusion complex characterization. Int. J. Pharm. 369, 5–11 (2009)
Shin, J.A., Lim, Y.G., Lee, K.H.: Copper-catalyzed azide–alkyne cycloaddition reaction in water using cyclodextrin as a phase transfer catalyst. J. Org. Chem. 77, 4117–4222 (2012)
Prasannan, A., Truong, T.L.B., Hong, P.D., Somanathan, N., Shown, I., Imae, T.: Synthesis and characterization of “hairy urchin”-like polyaniline by using β-cyclodextrin as a template. Langmuir 27, 766–773 (2010)
Shibu, E.S., Pradeep, T.: Quantum clusters in cavities: trapped Au15 in cyclodextrins. Chem. Mater. 23, 989–999 (2011)
Kida, T., Iwamoto, T., Asahara, H., Hinoue, T., Akashi, M.: Chiral recognition and kinetic resolution of aromatic amines via supramolecular chiral nanocapsules in nonpolar solvents. J. Am. Chem. Soc. 135, 3371–3374 (2013)
Taguchi, K.: Transient binding mode of phenolphthalein-β-cyclodextrin complex: an example of induced geometrical distortion. J. Am. Chem. Soc. 108, 2705–2709 (1986)
Acknowledgments
The work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 20625307), National Basic Research Program of China (973 Program, 2009CB930103) and Shandong Province Natural Science Foundation (No. ZR2009CL022).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yuehui Hou and Shangyang Li have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hou, Y., Li, S., Sun, T. et al. Organogels based on β-cyclodextrin system with molecular recognition property. J Incl Phenom Macrocycl Chem 80, 217–224 (2014). https://doi.org/10.1007/s10847-013-0379-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-013-0379-x