Abstract
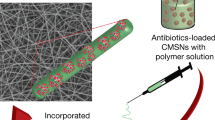
Nanofibrous materials have often been reported as carriers for clinical drugs but face the limitation of releasing the drugs in a burst fashion during use. The aim of this study is to produce composite nanofibrous mats with sustained release, using the broad spectrum antibiotic levofloxacin (LVF) as a model. Sustained release was achieved through two approaches, i.e. by firstly loading LVF into mesoporous silica nanoparticles (MSN) and then incorporating the MSN in the core regions of poly(ɛ-caprolactone) (PCL) nanofibres via core–shell electrospinning. Uniform PCL/LVF nanofibrous mats were also produced as controls. Loading of LVF into the MSN nanopores was confirmed by FTIR, BJH and BET measurements (100 mg LVF/g MSN). After electrospinning, electron microscopy revealed that the MSN were indeed confined in the core regions of the nanofibres. Drug release profiles showed that burst release was decreased from 59 % in the uniform PCL/LVF electrospun mats to 39 % in the core–shell PCL/LVF–MSN mats after 1 day in phosphate buffer at 37 °C, and gradual release in the latter was observed over the next 13 days. Antimicrobial assays showed that the composite electrospun mats were highly effective in killing Escherichia coli even after the mats had been incubated in a phosphate buffer for 14 days while the uniform PCL/LVF mats lost the ability after only 7 days. The results indicate that adsorption of the drug onto MSN and confining them in the core of nanofibres are effective ways of minimizing burst release and achieving sustained release of the drug.







Similar content being viewed by others
References
Langer R (1998) Drug delivery and targeting. Nature 392(6679 Suppl):5–10
De Jong WH, Borm PJ (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomed 3(2):133
Jain K (2008) Drug delivery systems—an overview. In: Jain K (ed) Drug delivery systems. Humana Press, Totowa, pp 1–50
Lavan DA, McGuire T, Langer R (2003) Small-scale systems for in vivo drug delivery. Nat Biotechnol 21(10):1184–1191
Ranade VV, Cannon JB (2011) Drug delivery systems. CRC Press, Boca Raton
Ramakrishna S et al (2005) An introduction to electrospinning and nanofibers, vol 90. World Scientific, Singapore
Wang X et al (2002) Electrospinning technology: a novel approach to sensor application. J Macromol Sci Part A 39(10):1251–1258
Huang Z-M et al (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63(15):2223–2253
Kenawy E-R et al (2009) Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater Chem Phys 113(1):296–302
Sill TJ, von Recum HA (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29(13):1989–2006
Zeng J et al (2003) Biodegradable electrospun fibers for drug delivery. J Control Release 92(3):227–231
Kenawy E-R et al (2007) Controlled release of ketoprofen from electrospun poly (vinyl alcohol) nanofibers. Mater Sci Eng A 459(1):390–396
He CL et al (2006) Coaxial electrospun poly (L-lactic acid) ultrafine fibers for sustained drug delivery. J Macromol Sci Part B 45(4):515–524
Zong X et al (2002) Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 43(16):4403–4412
Xiaoqiang L et al (2009) Fabrication and properties of core-shell structure P (LLA-CL) nanofibers by coaxial electrospinning. J Appl Polym Sci 111(3):1564–1570
Huang ZM et al (2006) Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J Biomed Mater Res Part A 77(1):169–179
Song W et al (2013) Coaxial PCL/PVA electrospun nanofibers: osseointegration enhancer and controlled drug release device. Biofabrication 5(3):035006
Yu D et al (2013) Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater 9(3):5665–5672
Qian W et al (2014) Dual drug release electrospun core-shell nanofibers with tunable dose in the second phase. Int J Mol Sci 15(1):774–786
Su Y et al (2011) Encapsulation and controlled release of heparin from electrospun poly (l-lactide-co-ε-caprolactone) nanofibers. J Biomater Sci Polym Ed 22(1–3):165–177
Wang C et al (2010) Biodegradable core/shell fibers by coaxial electrospinning: processing, fiber characterization, and its application in sustained drug release. Macromolecules 43(15):6389–6397
Meng ZX et al (2011) Fabrication, characterization and in vitro drug release behavior of electrospun PLGA/chitosan nanofibrous scaffold. Mater Chem Phys 125(3):606–611
Li L et al (2011) Electrospun poly (ɛ-caprolactone)/silk fibroin core-sheath nanofibers and their potential applications in tissue engineering and drug release. Int J Biol Macromol 49(2):223–232
Tarn D et al (2013) Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res 46(3):792–801
Liu X (2013) Fabrication of heparinized mesoporous silica nanoparticles as multifunctional drug carriers. J Chem 2013:430459
Grumezescu AM et al (2013) Biocompatible magnetic hollow silica microspheres for drug delivery. Curr Org Chem 17(10):1029–1033
Song B, Wu C, Chang J (2012) Dual drug release from electrospun poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles composite mats with distinct release profiles. Acta Biomater 8(5):1901–1907
Tsai C-H et al (2011) Surfactant-assisted controlled release of hydrophobic drugs using anionic surfactant templated mesoporous silica nanoparticles. Biomaterials 32(26):6234–6244
He Q, Shi J (2011) Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J Mater Chem 21(16):5845–5855
He Q et al (2009) Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles. Small 5(23):2722–2729
Yang P, Gai S, Lin J (2012) Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev 41(9):3679–3698
Kortesuo P et al (2000) Silica xerogel as an implantable carrier for controlled drug delivery—evaluation of drug distribution and tissue effects after implantation. Biomaterials 21(2):193–198
Slowing II et al (2008) Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev 60(11):1278–1288
Douroumis D (2011) Encapsulation of water insoluble drugs in mesoporous silica nanoparticles using supercritical carbon dioxide. J Nanomed Nanotechnol 2:111
Levine MM (1984) Escherichia coli infections. In: Germanier R (ed) Bacterial vaccines. Academic Press, Inc., New York, pp 187–235
Allen T (1997) Particle size measurement: volume 2: surface area and pore size determination, vol 2. Springer, New York
Zhang Y et al (2010) Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release 145(3):257–263
Shi Y-T et al (2010) The size-controllable synthesis of nanometer-sized mesoporous silica in extremely dilute surfactant solution. Mater Chem Phys 120(1):193–198
Middleton JC, Tipton AJ (2000) Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21(23):2335–2346
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32(8):762–798
Li Z-Z et al (2004) Fabrication of porous hollow silica nanoparticles and their applications in drug release control. J Control Release 98(2):245–254
Kanehata M, Ding B, Shiratori S (2007) Nanoporous ultra-high specific surface inorganic fibres. Nanotechnology 18(31):315602
Park H et al (2012) Fabrication of levofloxacin-loaded nanofibrous scaffolds using coaxial electrospinning. J Pharm Investig 42:1–5
Kim TG, Lee DS, Park TG (2007) Controlled protein release from electrospun biodegradable fiber mesh composed of poly (ɛ-caprolactone) and poly (ethylene oxide). Int J Pharm 338(1):276–283
Pawlak A, Mucha M (2003) Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta 396(1):153–166
Torres-Giner S et al (2012) Controlled delivery of gentamicin antibiotic from bioactive electrospun polylactide-based ultrathin fibers. Adv Eng Mater 14(4):B112–B122
Nguyen TTT, Chung OH, Park JS (2011) Coaxial electrospun poly(lactic acid)/chitosan (core/shell) composite nanofibers and their antibacterial activity. Carbohydr Polym 86(4):1799–1806
Lu F et al (2011) Effects of amphiphilic PCL–PEG–PCL copolymer addition on 5-fluorouracil release from biodegradable PCL films for stent application. Int J Pharm 419(1):77–84
McDonald PF et al (2010) In vitro degradation and drug release from polymer blends based on poly (dl-lactide), poly (l-lactide-glycolide) and poly (ε-caprolactone). J Mater Sci 45(5):1284–1292. doi:10.1007/s10853-009-4080-9
Acknowledgements
This work has been supported by CSIRO’s Manufacturing flagship. We would also like to thank Chi Huynh for her help with TEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalvandi, J., White, M., Truong, Y.B. et al. Release and antimicrobial activity of levofloxacin from composite mats of poly(ɛ-caprolactone) and mesoporous silica nanoparticles fabricated by core–shell electrospinning. J Mater Sci 50, 7967–7974 (2015). https://doi.org/10.1007/s10853-015-9361-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9361-x