Abstract
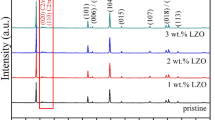
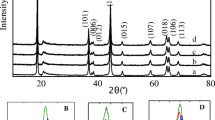
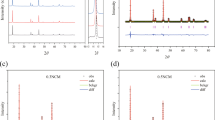
Lithium-rich manganese-based cathode materials have become one of the most concerned cathode materials for high-energy lithium-ion batteries. In order to improve its electrochemical performance, Li1.2Mn0.54Ni0.13Co0.13O2 with different content LiNbO3 coatings was synthesized by mechanical ball milling. The morphology, microstructure, and electrochemical properties of the samples were investigated by X-ray diffraction, scanning electron microscope, transmission electron microscope, galvanostatic charge/discharge, electrochemical impedance spectroscopy, and cyclic voltammetry. The results show that LiNbO3 coating not only protects the cathode material from the corrosion of electrolyte and HF but also improves the migration rate of Li+ in the interface region. Notably, the 5 wt% LiNbO3-coated Li1.2Mn0.54Ni0.13Co0.13O2 exhibits capacity retention of 89.9% under 0.1 C after 100 cycles. Besides, it has a higher discharge capacity than Li1.2Mn0.54Ni0.13Co0.13O2 at different rates. LiNbO3 coating is an effective way to improve its cycle stability and rate performance of Li1.2Mn0.54Ni0.13Co0.13O2.








Similar content being viewed by others
References
M.H. Sun, S.Z. Huang, L.H. Chen, Y. Li, X.Y. Yang, Z.Y. Yuan, B.L. Su, Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 45, 3479–3563 (2016)
W.D. Li, B.H. Song, A. Manthiram, High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017)
L. Kang, M. Zhang, J. Zhang, S. Liu, N. Zhang, W. Yao, Y. Ye, C. Luo, Z. Gong, C. Wang, X. Zhou, X. Wu, S.C. Jun, Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J. Mater. Chem. A 8, 24053–24064 (2020)
L. Kang, C. Huang, J. Zhang, M. Zhang, N. Zhang, S. Liu, Y. Ye, C. Luo, Z. Gong, C. Wang, X. Zhou, X. Wu, S.C. Jun, Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 390(2020)
S. Liu, L. Kang, J. Hu, E. Jung, J. Zhang, S.C. Jun, Y. Yamauchi, Unlocking the potential of oxygen-deficient copper-doped Co3O4 nanocrystals confined in carbon as an advanced electrode for flexible solid-state supercapacitors. ACS Energy Lett. 6, 3011–3019 (2021)
M.M. Thackeray, S.H. Kang, C.S. Johnson, J.T. Vaughey, R. Benedek, S.A. Hackney, Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112–3125 (2007)
H. Arai, S. Okada, Y. Sakurai, J. Yamaki, Reversibility of LiNiO2 cathode. Solid State Ion. 95, 275–282 (1997)
M. Gu, I. Belharouak, J.M. Zheng, H.M. Wu, J. Xiao, A. Genc, K. Amine, S. Thevuthasan, D.R. Baer, J.G. Zhang, N.D. Browning, J. Liu, C.M. Wang, Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 7, 760–767 (2013)
D. Mohanty, S. Kalnaus, R.A. Meisner, K.J. Rhodes, J.L. Li, E.A. Payzant, D.L. Wood, C. Daniel, Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction. J. Power Sources 229, 239–248 (2013)
M.K. Shobana, Metal oxide coated cathode materials for Li ion batteries—a review. J. Alloys Compd. 802, 477–487 (2019)
L. Zhou, Y.N. Wu, J. Huang, X. Fang, T. Wang, W.M. Liu, Y. Wang, Y. Jin, X.C. Tang, Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with Li+-conductive Li2SiO3 for lithium ion batteries. J. Alloys Compd. 724, 991–999 (2017)
L. He, J.M. Xu, T. Han, H. Han, Y.J. Wang, J. Yang, J.R. Wang, W.K. Zhu, C.J. Zhang, Y.H. Zhang, SmPO4-coated Li1.2Mn0.54Ni0.13Co0.13O2 as a cathode material with enhanced cycling stability for lithium ion batteries. Ceram. Int. 43, 5267–5273 (2017)
Y. Li, C. Wu, Y. Bai, L. Liu, H. Wang, F. Wu, N. Zhang, Y.F. Zou, Hierarchical mesoporous lithium-rich LiLi0.2Ni0.2Mn0.6O2 cathode material synthesized via ice templating for lithium-ion battery. ACS Appl. Mater. Interfaces 8, 18832–18840 (2016)
K. Redel, A. Kulka, A. Plewa, J. Molenda, High-performance Li-rich layered transition metal oxide cathode materials for Li-ion batteries. J. Electrochem. Soc. 166, A5333–A5342 (2019)
R.Z. Yu, Z.J. Zhang, S. Jamil, J.C. Chen, X.H. Zhang, X.Y. Wang, Z.H. Yang, H.B. Shu, X.K. Yang, Effects of nanofiber architecture and antimony doping on the performance of lithium-rich layered oxides: enhancing lithium diffusivity and lattice oxygen stability. ACS Appl. Mater. Interfaces 10, 16561–16571 (2018)
Y.B. Cao, X.Y. Qi, K.H. Hu, Y. Wang, Z.G. Gan, Y. Li, G.R. Hu, Z.D. Peng, K. Du, Conductive polymers encapsulation to enhance electrochemical performance of Ni-rich cathode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 10, 18270–18280 (2018)
G.R. Hu, X.Y. Qi, K.H. Hu, X.W. Lai, X. Zhang, K. Du, Z.D. Peng, Y.B. Cao, A facile cathode design with a LiNi0.6Co0.2Mn0.2O2 core and an AlF3-activated Li1.2Ni0.2Mn0.6O2 shell for Li-ion batteries. Electrochim. Acta 265, 391–399 (2018)
S.L. Pang, M. Zhu, K.J. Xu, X.Q. Shen, H.R. Wen, Y.J. Su, G.M. Yang, X. Wu, S.W. Li, W.Z. Wang, X.M. Xi, H.B. Wang, Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 via L-ascorbic acid-based treatment as cathode material for Li-ion batteries. J. Electrochem. Soc. 165, A1897–A1902 (2018)
L. Li, B.H. Song, Y.L. Chang, H. Xia, J.R. Yang, K.S. Lee, L. Lu, Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. J. Power Sources 283, 162–170 (2015)
X.D. Xiang, W.S. Li, Self-directed chemical synthesis of lithium-rich layered oxide Li Li0.2Ni0.2Mn0.6O2 with tightly interconnected particles as cathode of lithium ion batteries with improved rate capability. Electrochim. Acta 127, 259–265 (2014)
Q.Y. Wang, J. Liu, A.V. Murugan, A. Manthiram, High capacity double-layer surface modified LiLi0.2Mn0.54Ni0.13Co0.13O2 cathode with improved rate capability. J. Mater. Chem. 19, 4965–4972 (2009)
F. Wu, N. Li, Y.F. Su, L.J. Zhan, L.Y. Bao, J. Wang, L. Chen, Y. Zheng, L.Q. Dai, J.Y. Peng, S. Chen, Ultrathin spinel membrane-encapsulated layered lithium-rich cathode material for advanced Li-ion batteries. Nano Lett. 14, 3550–3555 (2014)
J.C. Zhang, R. Gao, L.M. Sun, Z.Y. Li, H. Zhang, Z.B. Hu, X.F. Liu, Understanding the effect of an in situ generated and integrated spinel phase on a layered Li-rich cathode material using a non-stoichiometric strategy. Phys. Chem. Chem. Phys. 18, 25711–25720 (2016)
T.F. Yi, Y.M. Li, S.Y. Yang, Y.R. Zhu, Y. Xie, Improved cycling stability and fast charge-discharge performance of cobalt-free lithium-rich oxides by magnesium-doping. ACS Appl. Mater. Interfaces 8, 32349–32359 (2016)
T.-F. Yi, X. Han, S.-Y. Yang, Y.-R. Zhu, Enhanced electrochemical performance of Li-rich low-Co Li1.2Mn0.56Ni0.16Co0.08–xAlxO2 (0≤x≤0.08) as cathode materials. Sci. China Mater. 59, 618–628 (2016)
T.-F. Yi, B. Chen, Y.-R. Zhu, X.-Y. Li, R.-S. Zhu, Enhanced rate performance of molybdenum-doped spinel LiNi0.5Mn1.5O4 cathode materials for lithium ion battery. J. Power Sources 247, 778–785 (2014)
S.Y. Yang, X.Y. Wang, X.K. Yang, Y.S. Bai, Z.L. Liu, H.B. Shu, Q.L. Wei, Determination of the chemical diffusion coefficient of lithium ions in spherical LiNi0.5Mn0.3Co0.2O2. Electrochim. Acta 66, 88–93 (2012)
C.S. Xu, H.T. Yu, C.F. Guo, Y. Xie, N. Ren, T.F. Yi, G.X. Zhang, Surface modification of Li1.2Mn0.54Ni0.13Co0.13O2 via an ionic conductive LiV3O8 as a cathode material for Li-ion batteries. Ionics 25, 4567–4576 (2019)
S.Q. Yang, P.B. Wang, H.X. Wei, L.B. Tang, X.H. Zhang, Z.J. He, Y.J. Li, H. Tong, J.C. Zheng, Li4V2Mn(PO4)4-stablized LiLi0.2Mn0.54Ni0.13Co0.13O2 cathode materials for lithium ion batteries. Nano Energy 63(2019)
J. Duan, W. Tang, R. Wang, X. Tang, J. Li, M. Tang, P. Li, Inhibited voltage decay and enhanced electrochemical performance of the Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material by CeAlOδ surface coating modification. Appl. Surf. Sci. 521(2020)
L. Zhao, Y. Sun, K. Song, F. Ding, Enhanced electrochemical performance of Li-rich Li[Li0.2Mn0.52Ni0.13Co0.13V0.02]O2 cathode materials for lithium ion batteries by Li1.13Mn0.47Ni0.2Co0.2O2 coating. Ionics 26, 4455–4462 (2020)
C. Chen, T.F. Geng, C.Y. Du, P.J. Zuo, X.Q. Cheng, Y.L. Ma, G.P. Yin, Oxygen vacancies in SnO2 surface coating to enhance the activation of layered Li-Rich Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for Li-ion batteries. J. Power Sources 331, 91–99 (2016)
X.D. Zhang, J.J. Hao, L.C. Wu, Z.M. Guo, Z.H. Ji, J. Luo, C.G. Chen, J.F. Shu, H.M. Long, F. Yang, A.A. Volinsky, Enhanced electrochemical performance of perovskite LaNiO3 coating on Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries. Electrochim. Acta 283, 1203–1212 (2018)
Y. Liu, Z. Yang, J. Zhong, J. Li, R. Li, Y. Yu, F. Kang, Surface-functionalized coating for lithium-rich cathode material to achieve ultra-high rate and excellent cycle performance. ACS Nano 13, 11891–11900 (2019)
Y.X. Hao, F.N. Yang, D.D. Luo, J.H. Tian, Z.Q. Shan, Improved electrochemical performances of yttrium oxyfluoride-coated LiLi0.2Mn0.54Ni0.13Co0.13O2 for lithium ion batteries. J. Energy Chem. 27, 1239–1246 (2018)
W.X. Zhang, Y.T. Liu, J.L. Wu, H.X. Shao, Y.F. Yang, Surface modification of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material with Al2O3/SiO2 composite for lithium-ion batteries. J. Electrochem. Soc. 166, A863–A872 (2019)
X. Nie, Z. Xu, L. Chen, J. Li, J. Cheng, Q. Sun, Y. Qiao, X. Xu, Y. Zhang, D. Li, R. Peng, L. Ci, Enhanced electrochemical performance of Li1.2[Mn0.54Co0.13Ni0.13]O2 enabled by synergistic effect of Li1.5Na0.5SiO3 modification. Adv. Mater. Interfaces 7, 2000378 (2020)
F. Yang, S.Z. Lin, Z.M. Guo, Y.R. Shao, B. Zhang, X.D. Zhang, S.H. Yan, A.A. Volinsky, Suppressed voltage decay and improved electrochemical performance by coating LiAl5O8 on the surface of Li1.2Mn0.54Ni0.13Co0.13O2. J. Alloys Compd. 805, 1034–1043 (2019)
Y.X. Zuo, B. Huang, C.M. Jiao, R.G. Lv, G.C. Liang, Enhanced electrochemical properties of LiLi0.2Mn0.54Ni0.13Co0.13O2 with ZrF4 surface modification as cathode for Li-ion batteries. J. Mater. Sci.-Mater. Electron. 29, 524–534 (2018)
C. Song, W. Feng, Z. Shi, Z. Huang, Coating TiO2 on lithium-rich Li1.2Mn0.54Ni0.13Co0.13O2 material to improve its electrochemical performance. Ionics 27, 457–468 (2020)
C.D. Li, Z.L. Yao, J. Xu, P. Tang, X. Xiong, Surface-modified LiLi0.2Mn0.54Ni0.13Co0.13O2 nanoparticles with LaF3 as cathode for Li-ion battery. Ionics 23, 549–558 (2017)
Funding
This work received support from the Chongqing Technology Innovation and Application Development project of Chongqing Science and Technology Commission (No.cstc2019jscx-msxmX0358), the Key Project of Science and Technology Research of Chongqing Education Commission of China (No.KJZDK201801103), the Scientific and Technological Research Foundation of Chongqing Municipal Education Commission (No.KJQN201901110), the Venture & Innovation Support Program for Chongqing Overseas Returnees (No.cx2019128), and the General program of Chongqing Natural Science Foundation (No.cstc2019jcyj-msxmX0165).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Wu, K., Xu, C. et al. LiNbO3-coated Li1.2Mn0.54Ni0.13Co0.13O2 as a cathode material with enhanced electrochemical performances for lithium-ion batteries. J Mater Sci: Mater Electron 32, 28223–28233 (2021). https://doi.org/10.1007/s10854-021-07199-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07199-1