Abstract
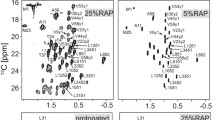
We describe the simplification of 13C–13C correlation spectra obtained from a microcrystalline protein sample expressed on a growth medium of 10% fully 13C labeled glucose diluted in 90% natural abundance glucose as compared to a fully labeled sample. Such a labeling scheme facilitates the backbone and side-chain resonance assignment of Phe, Tyr, His, Asp, Asn, Ile, Lys and Pro and yields an unambiguous stereospecific assignment of the valine Cγ1, Cγ2 13C resonances and of Leucine Cδ2.
Similar content being viewed by others
References
Böckmann A., Lange A., Galinier A., Luca S., Giraud N., Juy M., Heise H., Montserret R., Penin F., Baldus M. (2003) J. Biomol. NMR 27:323–39
Castellani F., van Rossum B., Diehl A., Schubert M., Rehbein K., Oschkinat H. (2002) Nature 420:98–102
Driscoll P.C., Gronenborn A.M., Clore G.M. (1989) FEBS Lett. 243:223–233
Folmer R.H., Hilbers C.W., Konings R.N., Nilges M. (1997) J. Biomol. NMR 9:245–258
Fung B.M., Khitrin A.K., Ermolaev K. (2000) J. Magn. Reson. 142:97–101
Gammeren A.J., Hulsbergen F.B., Hollander J.G., Groot H.J. (2005) J. Biomol. NMR 31:279–293
Grimmer A.R., Kretschmer A., Cajipe V.B. (1997) Magn. Reson. Chem. 35:86–90
Güntert P., Braun W., Billeter M., Wüthrich K. (1989) J. Am. Chem. Soc. 111:3997–4004
Havel T.F. (1991) Prog. Biophys. Mol. Biol. 56:43–78
Hediger S., Meier B.H., Ernst R.R. (1995) Chem. Phys. Lett. 240:449–456
Heise H., Seidel K., Etzkorn M., Becker S., Baldus M. (2005) J. Magn. Reson. 173:64–74
Igumenova T.I., McDermott A.E., Zilm K.W., Martin R.W., Paulson E.K., Wand A.J. (2004a) J. Am. Chem. Soc. 126:6720–6727
Igumenova T.I., Wand A.J., McDermott A.E. (2004b) J. Am. Chem. Soc. 126:5323–5331
Kanehisa M., Goto S., Kawashima S., Okuno Y., Hattori M. (2004) Nucleic Acids Res. 32:D277–D280
Keseler I.M., Collado-Vides J., Gama-Castro S., Ingraham J., Paley S., Paulsen I.T., Peralta-Gil M., Karp P.D. (2005) Nucleic Acids Res. 33:D334–D337
LeMaster D.M., Kushlan D.M. (1996) J. Am. Chem. Soc. 118:9255–9264
Pauli J., Baldus M., van Rossum B., de Groot H., Oschkinat H. (2001) Chembiochem 2:272–281
Sass J., Cordier F., Hoffmann A., Cousin A., Omichinski J.G., Lowen H., Grzesiek S. (1999) J. Am. Chem. Soc. 121:2047–2055
Senn H., Werner B., Messerle B.A., Weber C., Traber R., Wüthrich K. (1989) FEBS Lett. 249:113–118
Siemer A.B., Ritter C., Ernst M., Riek R., Meier B.H. (2005) Angew. Chem. Int. Ed. 44:2441–2444
Szyperski T., Neri D., Leiting B., Otting G., Wüthrich K. (1992) J. Biomol. NMR 2:323–334
Zech S.G., Wand A.J., McDermott A.E. (2005) J. Am. Chem. Soc. 127:8618–8626
Acknowledgements
We acknowledge scientific discussions with Matthias Ernst, Ansgar Siemer, Stephan Grzesiek, Hans-Jürgen Sass, David Sargent and Alvar Gossert. We thank Andreas Hunkeler and Urban Meier for technical assistance and Barth van Rossum for carefully reading the manuscript. The research was supported by the ETH Zurich (TH grant 30/03-1) and the Swiss National Science Foundation (SNF).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Schubert, M., Manolikas, T., Rogowski, M. et al. Solid-state NMR spectroscopy of 10% 13C labeled ubiquitin: spectral simplification and stereospecific assignment of isopropyl groups. J Biomol NMR 35, 167–173 (2006). https://doi.org/10.1007/s10858-006-9025-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-006-9025-x