Abstract
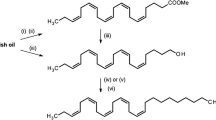
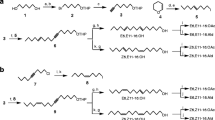
The type II class of sex pheromones found in moths is composed of polyene hydrocarbons and their epoxides. Analysis of Utetheisa ornatrix females by gas chromatography-mass spectrometry and measurement of responses of male moths by coupled gas chromatography-electroantennogram detection confirmed the presence of large amounts of (Z,Z,Z)-1,3,6,9-heneicosatetraene (1,3,6,9-21:Hy) and smaller amounts of (Z,Z,Z)-3,6,9-heneicosatriene (3,6,9-21:Hy). Both compounds were detected in pheromone glands of newly emerged adults, with low amounts found in the late pupal stage, indicating that sex pheromone biosynthesis started in the late pupal stage. In our population of females (several hundred sampled), approximately 90% produced the tetraene, 1,3,6,9-21:Hy, as the major component, while the other 10% produced only a large amount (1500–2000 ng) of 3,6,9-21:Hy, with no detectable amount of the tetraene. This result could indicate that two distinct populations are present in our original collection site in Florida. Decapitated female moths accumulated 3,6,9-21:Hy and 1,3,6,9-21:Hy compared to the same age normal females, indicating that female moths continuously produce pheromone. A pheromone biosynthesis activating neuropeptide (PBAN)-like neuropeptide did not affect sex pheromone production as indicated by injection of synthetic PBAN and decapitation of U. ornatrix female adults. When the labeled precursor, D4-9,12,15-18:acid, was injected into the early pupal stage, the most abundantly labeled hydrocarbons were 3,6,9-21:Hy and 1,3,6,9-21:Hy in the female adults. This result indicated that 3,6,9-21:Hy could be biosynthesized from linolenic acid through chain elongation and decarboxylation. To determine how 1,3,6,9-21:Hy is produced, D4-3,6,9-21:Hy was injected into pupae and monitored for incorporation of label. No label was incorporated into 1,3,6,9-21:Hy, although a large amount of triene, 3,6,9-21:Hy, was recovered in the pheromone gland. This indicates that U. ornatrix females do not use 3,6,9-21:Hy to produce 1,3,6,9-21:Hy, and the terminal double bond is introduced earlier in the biosynthetic pathway.





Similar content being viewed by others
References
Ando, T., Ohtani, K., Yamamoto, M., Miyamoto, T., Qin, X.-R., and Witjaksono. 1997. Sex pheromone of Japanese giant looper, Ascotis selenaria cretacea: identification and field tests. J. Chem. Ecol. 23:2413–2423.
Byer, J. A. 2006. Pheromone component patterns of moth evolution revealed by computer analysis of the Pherolist. J. Anim. Ecol. 75:399–407.
Choi, M. Y., Han, K. S., Boo, K. S., and Jurenka, R. A. 2002. Pheromone biosynthetic pathways in the moths Helicoverpa zea and Helicoverpa assulta. Insect Biochem. Mol. Biol. 32:1353–1359.
Conner, W. E. and Weller, S. J. 2004. A quest for alkaloids: the curious relationship between tiger moths and plants containing pyrrolizidine alkaloids, pp. 248–282, in R. T. Carde and J. G. Millar (eds.). Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge, UK.
Conner, W. E., Eisner, T. Vander Meer, R. K., Guerrero, A., Ghiringelli, D., and Meinwald, J. 1980. Sex attractant of an arctiid moth (Utetheisa ornatrix): a pulsed chemical signal. Behav. Ecol. Sociobiol. 7:55–63.
Del Campo, M. L. Smedley, S. R., and Eisner, T. 2005. Reproductive benefits derived from defensive plant alkaloid possession in an arctiid moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. U S A 102:13508–13512.
Diehl, P. A. 1975. Synthesis and release of hydrocarbons by the oenocytes of the desert locust, Schistocera gregaria. J. Insect Physiol. 21:1237–1246.
Dogra, V., Sabharwal, A., Sharma, S., Hug, M. A., Kad, G. L., and Vig, O. P. 1989. Synthesis of highly unsaturated insect sex pheromones. Synthesis of (3Z,6Z,9Z)-3,6,9-nonadecatriene and (3Z,6Z,9Z)-3,6,9-heneicosatriene. J. Indian Chem. Soc. 66:169–171.
Dussourd, D. E., Harvis, C. A., Meinwald, J., and Eisner, T. 1991. Pheromonal advertisement of a nuptial gift by a male moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. U S A 88:9224–9227.
Eisner, T. and Meinwald, J. 1995. The chemistry of sexual selection. Proc. Natl. Acad. Sci. U S A 92:50–55.
Grant, A. J., and O’connell, R. J. 2000. Responses of olfactory receptor neurons in Utetheisa ornatrix to gender-specific odors. J. Comp. Physiol. A 186:535–542.
Huang, W., Pulaski, S. P., and Meinwald, J. 1983. Synthesis of highly unsaturated insect pheromones: (Z,Z,Z)-1,3,6,9-heneicosatetraene and (Z,Z,Z)-1,3,6,9-nonadecatetraene. J. Org. Chem. 48:2270–2274.
Itagaki, H. and Conner, W. E. 1987. Neural control of rhythmic pheromone gland exposure in Utetheisa ornatrix (Lepidoptera: Arctiidae). J. Insect Physiol. 33:177–181.
Iyengar, V. K. and Eisner, T. 1999. Female choice increases offspring fitness in an arctiid moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. U S A 96:15013–15016.
Jain, S. C., Dussourd, D. E., Conner, W. E., Eisner, T. Guerrero, A., and Meinwald, J. 1983. Polyene pheromone components from an arctiid moth (Utetheisa ornatrix): characterization and synthesis. J. Org. Chem. 48:2266–2270.
Jeong, H.-S. 2003. Hydrocarbon pheromone biosynthesis of female velvetbean caterpillar moth, Anticarsia gemmatalis. MS dissertation. Iowa State University, Ames.
Jurenka, R. A. 2003. Biochemistry of female moth sex pheromones, pp. 53–80, in G. Blomquist and R. Vogt (eds.). Insect Pheromone Biochemistry and Molecular Biology. Academic Press, New York.
Jurenka, R. A., Subchev, M., Abad, J. L., Choi, M. Y., and Fabrias, G. 2003. Sex pheromone biosynthetic pathway for disparlure in the gypsy moth, Lymantria dispar. Proc. Natl. Acad. Sci. U S A 100:809–814.
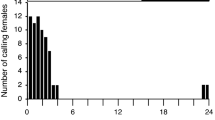
Lim, H. and Greenfield, M. D. 2007. Female pheromonal chorusing in an arctiid moth, Utetheisa ornatrix. Behav. Ecol. 18:165–173.
Lim, H., Park, K. C., Baker, T. C., and Greenfield, M. D. 2007. Perception of conspecific female pheromone stimulates female calling in an arctiid moth, Utetheisa ornatrix. J. Chem. Ecol. (in press).
Millar, J. G. 2000. Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annu. Rev. Entomol. 45:575–604.
Pease, R. W. J. 1968. Evolution and hybridization in the Utetheisa ornatrix complex (Lepidoptera: Arctiidae). I. Inter- and intra-population variation and its relation to hybridization. Evolution 22:719–735.
Rakoff, H. 1988. Preparation of methyl cis-9,cis-12,cis-15-octadecatrienoate-15,16-D2 and methyl cis-9,cis-12,cis-15-octadecatrienoate-6,6,7,7-D4. Lipids 23:280–285.
Schal, C., Sevala, V., and Carde, R. T. 1998. Novel and highly specific transport of a volatile sex pheromone by hemolymph lipophorin in moths. Naturwissenschaften 85:339–342.
Wei, W., Miyamoto, T., Endo, M., Murakawa, T., Pu, G.-Q., and Ando, T. 2003. Polyunsaturated hydrocarbons in the hemolymph: biosynthetic precursors of epoxy pheromones of geometrid and arctiid moths. Insect Biochem. Mol. Biol. 33:397–405.
Wei, W., Yamamoto, M., Asato, T., Fujii, T., Pu, G.-Q., and Ando, T. 2004. Selectivity and neuroendocrine regulation of the precursor uptake by pheromone glands from hemolymph in geometrid female moths, which secrete expoxyalkenyl sex pheromones. Insect Biochem. Mol. Biol. 34:1215–1224.
Witzgall, P., Lindblom, T. Bengtsson, M., and Toth, M. 2004. The Pherolist. ( http://www-pherolist.slu.se/pherolist.php ).
Acknowledgements
This research was, in part, supported by the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, project 6698.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, MY., Lim, H., Park, K.C. et al. Identification and Biosynthetic Studies of the Hydrocarbon Sex Pheromone in Utetheisa ornatrix . J Chem Ecol 33, 1336–1345 (2007). https://doi.org/10.1007/s10886-007-9306-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9306-1