Abstract
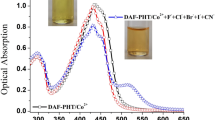
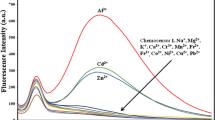
An amidine based chemosensor AM-1 was synthesized and characterized by various spectroscopic (FT-IR, 1H-NMR and mass) data and elemental analyses. Sensor AM-1 exhibited high selectivity and sensitivity towards Fe3+, Fe2+ and Cu2+ in the presence of other surveyed ions (such as Sr2+, Cr3+, Co2+, Ni2+, Zn2+, Ag+, Al3+, Ba2+, Ca2+, Cd2+, Cs+, Hg2+, K+, Li+, Mg2+, Mn2+, Na+ and Pb2+) with a distinct naked-eye detectable color change and a shift in the absorption band. Moreover, the emission of AM-1 was quenched selectively only in the presence of Fe3+.











Similar content being viewed by others
References
Bell TW, Hext NM (2004) Supramolecular optical chemosensors for organic analytes. Chem Soc Rev 33:589–598
Suksai C, Tuntulani T (2003) Luminescent chemodosimeters for bioimaging. Chem Soc Rev 32:192–202
Kuan Z, Yue LC, Ping HL, Fang L (2010) Chromo-chemodosimetric detection for Fe2+ by Fenton reagent-induced chromophore-decolorizing of halogenated phenolsulfonphthale in derivatives. Sci China Chem 53:1398–1405
Liang ZQ, Wang CX, Yang JX, Gao HW, Tian YP, Tao XT, Jiang MH (2007) A highly selective colorimetric chemosensor for detecting the respective amounts of iron(II) and iron(III) ions in water. New J Chem 31:906–910
Sen S, Sarkar S, Chattopadhyay B, Moirangthem A, Basu A, Dharad K, Chattopadhyay P (2012) A ratiometric fluorescent chemosensor for iron: discrimination of Fe2+ and Fe3+ and living cell application. Analyst 137:3335–3342
Bhalla V, Arora H, Kumar M (2013) Aggregates of a triphenylene based chemosensing ensemble for sensitive detection of cyanide ions in an aqueous medium. Dalton Trans 42:4450–4455
Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Silva AP, McCaughan B, McKinney BOF, Querol M (2003) Newer optical-based molecular devices from older coordination chemistry. Dalton Trans 10:1902–1913
Callan JF, Silva AP, Magri DC (2005) Luminescent sensors and switches in the early 21st century. Tetrahedron 61:8551–8588
Sahoo SK, Sharma D, Bera RK, Crisponi G, Callan JF (2012) Iron(III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 41:7195–7227
Crabtree RH (1994) Metal ions at work. Science 266:1591–1592
Burdo JR, Connor JR (2003) Brain iron uptake and homeostatic mechanisms: an overview. BioMetals 16:63–75
Pithadia AS, Lee MH (2012) Metal-associated amyloid-β species in Alzheimer’s disease. Curr Opin Chem Biol 16:67–73
High B, Bruce D, Richter MM (2001) Determining copper ions in water using electrochemilumincenec. Anal Chim Acta 449:17–22
Zhang LZ, Wang LY, Fan JL, Guo KX, Peng XJ (2011) A highly selective, fluorescent chemosensor for bioimaging of Fe3+. Bioorg Med Chem Lett 21:5413–5416
Brewer GJ (2008) Overview of the role of copper in neurodegenerative diseases and potential treatment with tetrathiomolybdate. Cell Biol Toxicol 24:423–426
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol 12:222–228
Li ZX, Zhang LF, Wang LN, Guo YK, Cai LH, Yu MM, Wei LH (2011) Highly sensitive and selective fluorescent sensor for Zn2+/Cu2+ and new approach for sensing Cu2+ by central metal displacement. Chem Commun 47:5798–5800
Jeong YS, Yoon JY (2012) Recent progress on fluorescent chemosensors for metal ions. Inorg Chim Acta 381:2–14
Shellaiah M, Wu YH, Singh A, Raju MVR, Lin HC (2013) Novel pyrene- and anthracene-based Schiff base derivatives as Cu2+ and Fe3+ fluorescence turn-on sensors and for aggregation induced emissions. J Mater Chem A1:1310–1318
Li J, Hu Q, Yu X, Zeng Y, Cao C, Liu X, Guo J, Pan Z (2011) A novel rhodamine-benzimidazole conjugate as a highly selective turn-on fluorescent probe for Fe3+. J Fluoresc 21:2005–2013
Hu ZQ, Wang XM, Feng YC, Ding L, Li M, Lin CS (2011) A novel colorimetric and fluorescent chemosensor for acetate ions in aqueous media based on a rhodamine 6G–phenylurea conjugate in the presence of Fe(III) ions. Chem Commun 47:1622–1624
Yang Z, Yan C, Chen Y, Zhu C, Zhang C, Dong X, Yang W, Guo Z, Lu Y, He W (2011) A novel terpyridine/benzofurazan hybrid fluorophore: metal sensing behavior and application. Dalton Trans 40:2173–2176
Pathak RK, Hinge VK, Mondala P, Rao CP (2012) Ratiometric fluorescence off-on-off sensor for Cu2+ in aqueous buffer by a lower rim triazole linked benzimidazole conjugate of calix[4]arene. Dalton Trans 41:10652–10660
Singh N, Mulrooney RC, Kaur N, Callan JF (2008) A nanoparticle based chromogenic chemosensor for the simultaneous detection of multiple analytes. Chem Commun 4900–4902
Jiang Z, Tang L, Shao F, Zheng G, Lu P (2008) Synthesis and characterization of 9-(cycloheptatrienylidene) fluorene derivatives: new fluorescent chemosensors for detection of Fe3+ and Cu2+. Sensors Actuators B 134:414–418
Zhao XJ, He RX, Li M, Zhao NW, Li YF, Huang CZ (2013) Formation of blue fluorescent ribbons of 4′,4″″-(1,4-phenylene)bis(2′,:6′,2″-terpyridine) and highly selective visual detection of iron(II) cations. RSC Adv 3:111–116
Patil SR, Nandre JP, Jadhav D, Bothra S, Sahoo SK, Devi M, Pradeep CP, Mahulikar PP, Patil UD (2014) Imatinib intermediate as a two in one dual channel sensor for the recognition of Cu2+ and I‾ ions in aqueous media and its practical applications. Dalton Trans. 43:13299–13306
Nandre J, Patil S, Patil V, Yu F, Chen L, Sahoo S, Prior T, Redshaw C, Mahulikar P, Patil U (2014) A novel fluorescent “turn-on” chemosensor for nanomolar detection of Fe(III) from aqueous solution and its application in living cells imaging. Biosens Bioelectron 61:612–617
Sharma D, Moirangthem A, Sahoo SK, Basu A, Roy SM, Pati RK, Kumar ASK, Nandred JP, Patil UD (2014) Anion selective chromogenic and fluorogenic chemosensor and its application in breast cancer live cell imaging. RSC Adv 4:41446–41452
Chandel M, Roy SM, Sharma D, Sahoo SK, Patel A, Kumari P, Dhale RS, Ashok KSK, Nandre JP, Patil UD (2014) Anion recognition ability of a novel azo dye derived from 4-hydroxycoumarin. J Lumin 154:515–519
Sharma D, Sahoo SK, Chaudhary S, Bera RK, Callan JF (2013) Fluorescence ‘turn-on’ sensor for F− derived from vitamin B6 cofactor. Analyst 138:3646–3650
Patil UD, Mahulikar PP (2012) A convenient, TiCl4/SnCl4 mediated synthesis of N-phenyl or N-aryl benzamidines and N-phenyl picolinamidines. ISRN Organic Chemistry Article ID 963195, 1-6; doi:10.5402/2012/963195
Stern O, Volmer M (1919) Uber die abklingungszeit der fluoreszenz. Phys J 20:183–188
Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino F, Zheng G, Sonnenberg GJ, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JN, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian, Inc., Wallingford
Acknowledgments
The author Dr. U. D. Patil is grateful to Department of Science & Technology, New Delhi, INDIA (Reg. No. CS-088/2013) and ‘North Maharashtra University, Jalgaon’, VCRMS Scheme, for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2.19 MB)
Rights and permissions
About this article
Cite this article
Nandre, J., Patil, S., Patil, P. et al. The Amidine Based Colorimetric Sensor for Fe3+, Fe2+, and Cu2+ in Aqueous Medium. J Fluoresc 24, 1563–1570 (2014). https://doi.org/10.1007/s10895-014-1438-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1438-4