Abstract
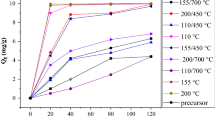
In this paper, the gelation technique was utilized for the synthesis of a Si/Cu amorphous sample composed of oxygen, sodium, silicon, and copper. The obtained sample was identified utilizing FT-IR, EDS, XRD, and FE-SEM instruments. The non-crystalline nature of the obtained sample was confirmed through the presence of XRD broadband in the range 2θ = 16°–46°. Also, the obtained sample was utilized as an economically inexpensive and efficient adsorbent for the removal of methylene blue dye from aqueous media. Besides, several parameters were examined for evaluating the removal of methylene blue dye, for example, kinetic, equilibrium, thermodynamic, and reusability. Moreover, the Freundlich isotherm and pseudo-first-order kinetic model controlled the adsorption process. The maximum adsorption capacity of the obtained adsorbent was 102.05 mg/g. In addition, the negative ∆G° values affirmed the spontaneous properties of the adsorption. Also, the adsorption was exothermic and physical because ∆H° is negative and less than 40 kJ/mol. Finally, the obtained adsorbent was regenerated then reused several times without changing their removing efficiency.











Similar content being viewed by others
References
M.E. Mahmoud, G.M. Nabil, M.A. Khalifa, N.M. El-mallah, H.M. Hassouba, Effective removal of crystal violet and methylene blue dyes from water by surface functionalized zirconium silicate nanocomposite. J. Environ. Chem. Eng. 7, 103009 (2019)
S. Khe, M. Ching, N. Ling, Effect of ultrasound pre-treatment on adsorbent in dye adsorption compared with ultrasound simultaneous adsorption. Ultrason. Sonochem. 48, 64–70 (2018)
M. Rafatullah, O. Sulaiman, R. Hashim, A. Ahmad, Adsorption of methylene blue on low-cost adsorbents: a review. J. Hazard. Mater. 177, 70–80 (2010)
S. Bentahar, A. Dbik, M. El Khomri, N. El Messaoudi, Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 5, 5921–5932 (2017)
S. Chatterjee, S. Lim, S.H. Woo, Removal of Reactive Black 5 by zero-valent iron modified with various surfactants. Chem. Eng. J. 160, 27–32 (2010)
Y. Miyah, A. Lahrichi, M. Idrissi, A. Khalil, F. Zerrouq, Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: equilibrium and kinetic studies. Surf. Interfaces 11, 74–81 (2018)
N. Nikooe, E. Saljoughi, Preparation and characterization of novel PVDF nanofiltration membranes with hydrophilic property for filtration of dye aqueous solution. Appl. Surf. Sci. 413, 41–49 (2017)
M.E. Osugi, K. Rajeshwar, E.R.A. Ferraz, D.P. De Oliveira, Â.R. Araújo, M. Valnice, B. Zanoni, Comparison of oxidation efficiency of disperse dyes by chemical and photoelectrocatalytic chlorination and removal of mutagenic activity. Electrochim. Acta 54, 2086–2093 (2009)
T. Kim, C. Park, J. Yang, S. Kim, Comparison of disperse and reactive dye removals by chemical coagulation and Fenton oxidation. J. Hazard. Mater. 112, 95–103 (2004)
X. Li, S. Tang, D. Yuan, J. Tang, C. Zhang, N. Li, Y. Rao, Improved degradation of anthraquinone dye by electrochemical activation of PDS. Ecotoxicol. Environ. Saf. 177, 77–85 (2019)
L. Gui, J. Peng, P. Li, R. Peng, P. Yu, Y. Luo, Electrochemical degradation of dye on TiO2 nanotube array constructed anode. Chemosphere 235, 1189–1196 (2019)
S. Sharma, N. Khare, Hierarchical Bi2S3 nanoflowers: a novel photocatalyst for enhanced photocatalytic degradation of binary mixture of Rhodamine B and Methylene blue dyes and degradation of mixture of p-nitrophenol and p-chlorophenol. Adv. Powder Technol. 29, 3336–3347 (2018)
X. Xie, N. Liu, F. Yang, Q. Zhang, X. Zheng, Y. Wang, Comparative study of antiestrogenic activity of two dyes after Fenton oxidation and biological degradation. Ecotoxicol. Environ. Saf. 164, 416–424 (2018)
J. Wu, L. Ma, Y. Chen, Y. Cheng, Y. Liu, X. Zha, Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: removal and pathways. Water Res. 92, 140–148 (2016)
S. Maria, D.A. Guelli, U. De Souza, K. Angela, S. Bonilla, A. Augusto, U. De Souza, Removal of COD and color from hydrolyzed textile azo dye by combined ozonation and biological treatment. J. Hazard. Mater. 179, 35–42 (2010)
V. Katheresan, J. Kansedo, S.Y. Lau, Efficiency of various recent wastewater dye removal methods: a review. J. Environ. Chem. Eng. 6, 4676–4697 (2018)
H. Mittal, S.M. Alhassan, S. Sinha, Efficient organic dye removal from wastewater by magnetic carbonaceous adsorbent prepared from corn starch. J. Environ. Chem. Eng. 6, 7119–7131 (2018)
G. Patra, R. Barnwal, S.K. Behera, B.C. Meikap, Removal of dyes from aqueous solution by sorption with fly ash using a hydrocyclone. J. Environ. Chem. Eng. 6, 5204–5211 (2018)
E.A. Moawed, A.E. Wahba, R.A. Gabr, Synthesis and application of LGB/St/Al2O3 biocomposite for sensitive detection and efficient removal of brilliant green dye from wastewater. J. Environ. Chem. Eng. 6, 7225–7232 (2018)
L. Aljerf, High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: kinetics and equilibrium study. J. Environ. Manag. 225, 120–132 (2018)
E.A. Abdelrahman, R.M. Hegazey, R.E. El-azabawy, Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents, J. Mater. Res. Technol. https://doi.org/10.1016/j.jmrt.2019.08.051
C. Arora, S. Soni, S. Sahu, J. Mittal, P. Kumar, P.K. Bajpai, Iron based metal organic framework for efficient removal of methylene blue dye from industrial waste. J. Mol. Liq. 284, 343–352 (2019)
S. Lapwanit, T. Sooksimuang, T. Trakulsujaritchok, Adsorptive removal of cationic methylene blue dye by kappa-carrageenan/poly(glycidyl methacrylate) hydrogel beads: preparation and characterization. J. Environ. Chem. Eng. 6, 6221–6230 (2018)
W.A. Khanday, M. Asif, B.H. Hameed, Cross-linked beads of activated oil palm ash zeolite/chitosan composite as a bio-adsorbent for the removal of methylene blue and acid blue 29 dyes. Int. J. Biol. Macromol. 95, 895–902 (2017)
M. Wang, R. Xie, Y. Chen, X. Pu, W. Jiang, L. Yao, A novel mesoporous zeolite-activated carbon composite as an effective adsorbent for removal of ammonia-nitrogen and methylene blue from aqueous solution. Bioresour. Technol. 268, 726–732 (2018)
D.M. El-mekkawi, F.A. Ibrahim, M.M. Selim, Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. Biochem. Pharmacol. 4, 1417–1422 (2016)
N.H. Othman, N.H. Alias, M.Z. Shahruddin, N. Fitrah, A. Bakar, N. Raikhan, N. Him, W. Jye, Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide. J. Environ. Chem. Eng. 6, 2803–2811 (2018)
H.M. Aly, M.E. Moustafa, E.A. Abdelrahman, Influence of aluminum source on the synthesis of nanosized ZSM-5 zeolite. Der Chem. Sin. 4, 68–72 (2013)
E.A. Abdelrahman, R.M. Hegazey, Exploitation of Egyptian insecticide cans in the fabrication of Si/Fe nanostructures and their chitosan polymer composites for the removal of Ni(II), Cu(II), and Zn(II) ions from aqueous solutions. Composites B 166, 382–400 (2019)
E.A. Abdelrahman, R.M. Hegazey, Utilization of waste aluminum cans in the fabrication of hydroxysodalite nanoparticles and their chitosan biopolymer composites for the removal of Ni(II) and Pb(II) ions from aqueous solutions: kinetic, equilibrium, and reusability studies. Microchem. J. 145, 18–25 (2019)
E.A. Abdelrahman, Synthesis of zeolite nanostructures from waste aluminum cans for efficient removal of malachite green dye from aqueous media. J. Mol. Liq. 253, 72–82 (2018)
E.A. Abdelrahman, E.T. Abdel-salam, S.M. El Rayes, N.S. Mohamed, Facile synthesis of graft copolymers of maltodextrin and chitosan with 2-acrylamido-2-methyl-1-propanesulfonic acid for efficient removal of Ni(II), Fe(III), and Cd(II) ions from aqueous media, J. Polym. Res. (2019) https://doi.org/10.1007/s10965-019-1920-4
K. Rida, S. Bouraoui, S. Hadnine, Adsorption of methylene blue from aqueous solution by kaolin and zeolite. Appl. Clay Sci. 83–84, 99–105 (2013)
D. Ghosh, K.G. Bhattacharyya, Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 20, 295–300 (2002)
K. He, G. Chen, G. Zeng, A. Chen, Z. Huang, J. Shi, M. Peng, T. Huang, L. Hu, Journal of the Taiwan Institute of Chemical Engineers enhanced removal performance for methylene blue by kaolin with graphene oxide modification. J. Taiwan Inst. Chem. Eng. 89, 77–85 (2018)
J. Gong, B. Wang, G. Zeng, C. Yang, C. Niu, Q. Niu, Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 164, 1517–1522 (2009)
E. Nazarzadeh, A. Motahari, M. Sillanpää, Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: a review. Environ. Res. 162, 173–195 (2018)
D. Kavitha, C. Namasivayam, Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 98, 14–21 (2007)
Acknowledgements
The author would like to thank the Deanship of Scientific Research at Umm Al-Qura University for the continuous support. This work was supported financially by the Deanship of Scientific Research at Umm Al-Qura University (Grant Code 18-SCI-1-02-0005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hameed, A.M. Synthesis of Si/Cu Amorphous Adsorbent for Efficient Removal of Methylene Blue Dye from Aqueous Media. J Inorg Organomet Polym 30, 2881–2889 (2020). https://doi.org/10.1007/s10904-019-01436-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01436-1