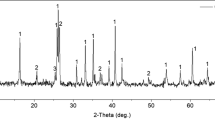
Abstract
Power plant ash was used to synthesize new materials by hydrothermal treatment and ultrasonic activation in an alkaline medium (5 M NaOH) under moderate experimental conditions. The obtained materials were characterized by a variety of techniques (SEM/EDS, INAA, XRD, FTIR, BET). Preliminary investigation of the Ba, Cd, Cr, Cs, Eu and U removal efficiency was performed by batch experiments using radiotracers and spectroscopic techniques as well as desorption studies. The results indicated that the new materials are very efficient in removing the above mentioned metals from aqueous solutions and can be considered as potential low-cost sorbents in environmental technology.








Similar content being viewed by others
References
Reijnders L (2005) Disposal, uses and treatments of combustion ashes: a review. Resour Conserv Recycl 43:313–336
Lokeshappa B, Dikshit AK, International Conference on Life Science and Technology IPCBEE vol. 3, 2011 IACSIT Press, Singapore (http://www.ipcbee.com/vol3/4-L009.pdf, Assessed on 1.10.2014)
Ahmaruzzaman A (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36:327–363
Fan Y, Zhang FS, Zhu J, Liu Z (2008) Effective utilization of waste ash from MSW and coal co-combustion power plant-Zeolite synthesis. J Hazard Mater 153:382–388
Wang S, Wu H (2006) Environmental-benign utilisation of fly ash as low-cost adsorbents. J Hazard Mater B136:482–501
Hernandez-Ramirez O, Holmes SM (2008) Novel and modified materials for wastewater treatment applications. J Mater Chem 18:2751–2761
Izidoro JC, Fungaro DA, Abbott JE, Wang S (2013) Synthesis of zeolites X and A zeolite from fly ash for cadmium and zinc removal from aqueous solutions in single and binary ion systems. Fuel 103:827–834
Chiang YW, Ghyselbrecht K, Santos RM, Meesschaert B, Martens JA (2012) Synthesis of zeolitic-type adsorbent materials from municipal solid waste incinerator bottom ash and its application in heavy metal adsorption. Catal Today 190:23–30
Mumpton FA (1999) La roca magica: uses of natural zeolites in agriculture and industry. Proc Natl Acad Sci USA 96:3463–3470
Misaelides P (2011) Application of natural zeolites in environmental remediation: a short review. Micropor Mesopor Mater 144:15–18
El-Naggar MR, El-Kamash AM, Dessouky MI, Ghonaim AK (2008) Two step method for preparation of NaA-X zeolite blend from fly ash for removal of cesium ions. J Hazard Mat 154:963–972
Asl SMH, Ahmadi M, Ghiasvand M, Tardast A, Katal R (2013) Artificial neural network (ANN) approach for modeling of Cr(VI) adsorption from aqueous solution by zeolite prepared from raw fly ash (ZFA). J Ind Eng Chem 19:1044–1055
Gupta VK, Carrott PJM, Ribeiro M, Suhas ML (2009) Low-cost adsorbents: growing approach to wastewater treatment-a review. Crit Rev Environ Sci Technol 39:783–842
Harja M, Buema G, Sutiman DM, Munteanu C, Bucur D (2012) Low cost adsorbents obtained from ash for copper removal. Korean J Chem Eng 29:1735–1740
Harja M, Buema G, Sutiman DM, Gretescu I (2013) Removal of heavy metal ions from aqueous solutions using low-cost sorbents obtained from ash. Chem Pap 67:497–508
Murayama N, Yamamoto H, Shibata J (2002) Mechanism of zeolite synthesis from coal fly ash by alkali activation. Int J Miner Process 64:1–17
Molina A, Poole C (2004) A comparative study using two methods to produce zeolites from fly ash. Miner Eng 17:167–173
Musyoka N, Petrik L, Hums E, Baser H, Schwieger W (2012) In situ ultrasonic monitoring of zeolite A crystallization from coal fly ash. Catal Today 190:38–46
Rasouli M, Yaghobi N, Hafezi M (2012) Adsorption of divalent lead ions from aqueous solution using low silica nano-zeolite X. J Ind Eng Chem 18:1970–1976
Ryu TG, Ryu JC, Choi CH, Kim CG, Yoo SJ, Yang HS, Kim YH (2006) Preparation of Na–P1 zeolite with high cation exchange capacity from coal fly ash. J Ind Eng Chem 12:401–407
Um NI, Han GC, You KS, Ahn JW (2009) Immobilization of Pb, Cd and Cr by synthetic NaP1 zeolites from coal bottom ash treated by density separation. Resour Process 56:130–136
Gross M, Soulard M, Caullet P, Patarin J, Saude I (2007) Synthesis of faujasite from coal fly ashes under smooth temperature and pressure conditions: a cost saving process. Micropor Mesopor Maters 104:67–76
Wang CF, Li JS, Wang LJ, Sun XY (2008) Influence of NaOH concentrations on synthesis of pure-form zeolite A from fly ash using two-stage method. J Hazard Mater 155:58–64
Komljenovic M, Bascarevic Z, Bradic V (2010) Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J Hazard Mater 181:35–42
Deyi W, Yanming S, Shengbing H, Xinze W, Chunjie L, Hainan K (2008) Removal of trivalent chromium from aqueous solution by zeolite synthesized from coal fly ash. J Hazard Mater 155:415–423
Mishra T, Tiwari SK (2006) Studies on sorption properties of zeolite derived from Indian fly ash. J Hazard Mater B137:299–303
Javadian H, Ghorbani F, Tayebi HA, Asl SMH (2013) Study of the adsorption of Cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arab J Chem 1:1–13
Jha VK, Matsuda M, Miyake M (2008) Sorption properties of the activated carbon-zeolite composite prepared from coal fly ash for Ni2+, Cu2+, Cd2+ and Pb2+. J Hazard Mater 160:148–153
Medina A, Gamero P, Almanza JM, Vargas A, Montoya A, Vargas G, Izquierdo M (2010) Fly ash from a Mexican mineral coal. II. Source of W zeolite and its effectiveness in arsenic (V) adsorption. J Hazard Mater 181:91–104
Toxicological profile for cadmium (2012) Agency for toxic substances and disease registry (ATSDR), U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA
Toxicological profile for chromium (2012) Agency for toxic substances and disease registry (ATSDR), U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA
Toxicological profile for uranium (2013) Agency for toxic substances and disease registry (ATSDR), U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA
Toxicological profile for cesium (2004) Agency for toxic substances and disease registry (ATSDR), U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA
Toxicological profile for barium (2007) Agency for toxic substances and disease registry (ATSDR), U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA
Pankaj S, Radha T (2011) Sorption behaviour of nanocrystalline MOR type zeolite for Th(IV) and Eu(III) removal from aqueous waste by batch treatment. J Colloid Interface Sci 362:144–156
Shao DD, Fan QH, Li JX, Niu ZW, Wuc WS, Chen YX, Wanga XK (2009) Removal of Eu(III) from aqueous solution using ZSM-5 zeolite. Micropor Mesopor Mater 123:1–9
Baeza-Alvarado MD, Olguín MT (2011) Surfactant-modified clinoptilolite-rich tuff to remove barium (Ba2+) and fulvic acid from mono- and bi-component aqueous media. Micropor Mesopor Mater 139:81–86
Buema G, Noli F, Misaelides P, Harja M, Sutiman DM, Cretescu I (2013) Uranium removal from aqueous solutions by raw and modified thermal power plant ash. J Radioanal Nucl Chem 299:381–386
EPA Test Method 1311–TCLP
Medina A, Gamero P, Querol X, Moreno N, De Leon B, Almanza M, Vargas G, Izquierdo M, Font O (2010) Fly ash from a Mexican mineral coal I: mineralogical and chemical Characterization. J Hazard Mater 181:82–90
Mozgawa W (2001) The relation between structure and vibrational spectra of natural zeolites. J Mol Struct 596:129–136
Shigemoto N, Sugiyama S, Hayashi H, Miyaura K (1995) Characterization of Na-X and coal fly ash zeolites and their amorphous precursors by IR, MAS NMR and XPS. J Mater Sci 30:5777–5783
Mainganye D, Ojumu TV, Petrik L (2013) Synthesis of zeolites Na-P1 from South African Coal Fly Ash: effect of impeller design and agitation. Materials 6:2074–2089
Yildiz B, Erten HN, Kis M (2011) The sorption behavior of Cs+ ion on clay minerals and zeolite in radioactive waste management:sorption kinetics and thermodynamics. J Radioanal Nucl Chem 288:475–483
Pena Penilla R, Guerrero Bustos A, Goni Elizalde S (2006) Immobilization of Cs, Cd, Pb and Cr by synthetic zeolites from Spanish low-calcium coal fly ash. Fuel 85:823–832
Borai EH, Harjula R, Malinen L, Paajanen A (2009) Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J Hazard Mater 172:416–422
Faghihian H, Iravani M, Moayed M, Ghannadi-Maragheh M (2013) Preparation of a novel PAN-zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solutions: kinetic, equilibrium, and thermodynamic studies. Chem Eng J 222:41–48
Visa M, Chelaru AM (2014) Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl Surf Sci 303:14–22
Papandreou AD, Stournaras CJ, Panias D, Paspaliaris I (2011) Adsorption of Pb(II), Zn(II) and Cr(III) on coal fly ash porous pellets. Miner Eng 24:1495–1501
Belgacem A, Rediai R, Hadoun H, Khemaissia S, Belmedani M (2014) The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ Sci Pollut Res 21:684–694
Van Roy S, Vanbroekhoven K, Dejonghe W, Diels L (2006) Immobilization of heavy metals in the saturated zone by sorption and in situ bioprecipitation processes. Hydrometallurgy 83:195–203
Acknowledgments
This paper was performed with the support of POSDRU CUANTUMDOC “Doctoral Studies for European Performances in Research and Inovation” ID79407 Project funded by the European Social Found and Romanian Government. The authors would like to acknowledge the staff at the School of Science of the Aristotle University of Thessaloniki for the SEM/EDS, IR and BET measurements and the staff of the reactor TU-Delft Institute, The Netherlands (NMI3-II Grant No. 283883) for the INAA measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noli, F., Buema, G., Misaelides, P. et al. New materials synthesized from ash under moderate conditions for removal of toxic and radioactive metals. J Radioanal Nucl Chem 303, 2303–2311 (2015). https://doi.org/10.1007/s10967-014-3762-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3762-1