Abstract
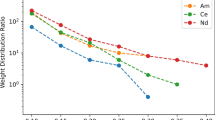
The separation of americium(III) from europium(III) was achieved utilizing a bis-2,6-(5,6,7,8-tetrahydro-5,9,9-trimethyl-5,8-methano-1,2,4-benzotriazin-3-yl) pyridine (CA-BTP) chromatographic resin. The extraction chromatographic materials were prepared using various concentrations of CA-BTP. This new, hydrolytically stable extractant was impregnated on an inert polymeric support at 40% loading. The uptake of Am(III) and Eu(III) by this material from 0.1 to 4.0 M aqueous HNO3 solutions was measured. The resulting dry weight distribution ratios, D w , indicated a strong preference for Am(III) with little affinity for Eu(III). These results are similar to recently reported solvent extraction studies indicating a maximum uptake of Am(III) in the 0.5–1.0 M HNO3 range. The resin preparation, performance, and characterization of the Am/Eu separation are reported herein.






Similar content being viewed by others
References
Mathur J, Murali M, Nash K (2001) Actinide partitioning—a review. Solvent Extr Ion Exch 19:357–390
Nash K, Madic C, Mathur J, Lacquement J (2007) Actinide separation science and technology. In: Bond AH, Dietz ML, Rogers RD (eds) The chemistry of the actinide and the transactinide elements, vol 4. Springer, Dordrecht, pp 2622–2798
Strategy for the management and disposal of used nuclear fuel and high-level radioactive waste. Department of Energy. January 2013
Magill J, Berthou V, Haas D, Galy J, Schenkel R, Wiese H-W, Heusener G, Thommasi J, Youinou G (2003) Impact limits of partitioning and transmutation scenarios on the radiotoxicity of actinides in radioactive waste. Nucl Energy 42:263–277
Mayer K, Wallenius M, Ray I (2005) Nuclear forensics—a methodology providing clues on the origin of illicitly trafficked nuclear materials. Analyst 130:433–441
Panak P, Geist A (2013) Complexation and extraction of trivalent actinides by triazinylpyridine N-donor ligands. Chem Rev 113:1199–1236
Musikas C, Le Marois G, Fitoussi R, Cuillerdier C (1980) Properties and uses of nitrogen and sulfur donors ligands in actinide separations. ACS Symposium Series. American Chemical Society, Washington, DC
Kurosaki H, Clark SB (2011) Chromatographic separation of Am and Cm. Radiochim Acta 99:65–69
Kolarik Z, Müllich U, Gassner F (1999) Selective extraction of Am(III) over Eu(III) by 2,6-ditriazolyl- and 2,6-ditriazinylpyridines. Solvent Extr Ion Exch 17:23–32
Kolarik Z, Müllich U, Gassner F (1999) Extraction of Am(III) and Eu(III) nitrates by 2-6-di-(5,6-dipropyl-1,2,4-triazin-3-yl)pyridines. Solvent Extr Ion Exch 17:1155–1170
Muraview D, Ghantous L, Valiente M (1998) Stabilization of solvent-impregnated resin capacities by different techniques. React Funct Polym 38:259–268
Tevepaugh K, Carrick J, Tai S, Coonce JG, Delmau LH, Ensor DD (2016) Separation of americium from europium using camphor-bistriazinyl pyridine: a fundamental study. Solvent Extr Ion Exch 34(1):13–25
Wei Y-Z, Hoshi H, Kumagai M, Asakura T, Morita Y (2004) Separation of Am(III) and Cm(III) from trivalent lanthanides by 2,6-bistriazinylpyridine extraction chromatography for radioactive waste management. J Alloys Compd 374:447–450
Deepika P, Sabharwal KN, Srinivasan TG, Vasudeva Rao PR (2012) Studies on separation of minor actinides from lanthanides from high level waste by extraction chromatography using 2,6-bistriazinylpyridine. Nucl Technol 179:407–416
Klug C, Sudowe R (2013) A novel extraction chromatography resin for trivalent actinides using 2,6-bis(5,6-diisobutyl-1,2,4-triazine-3-yl)pyridine. Sep Sci Technol 48(17):2567–2575
Hill C, Guillaneux D, Berthon L, Madic CJ (2002) Sanex-BTP process development studies. Nucl Sci Technol 39(Suppl. 3):309–312
Kolarik Z (2008) Complexation and separation of lanthanides(III) and actinides(III) by heterocyclic N-donors in solution. Chem Rev 108:4208–4252
Hudson MJ, Boucher CE, Braekers D, Desreux JF, Drew MGB, Foreman MRS, Harwood LM, Hill C, Madic C, Marken F, Youngs TGA (2006) New bis(triazinyl) pyridines for selective extraction of americium(III). New J Chem 30:1171–1183
Deepika P, Sabharwal KN, Srinivasan TG, Vasudeva Rao PR (2013) Studies on bis annulated triazinyl pyridine (BATP) using extraction chromatography. Sep Sci Technol 48(13):2020–2027
Madic C, Testard F, Liljenzin J-O, Christiansen B, Ferrando M, Facchini A, Geist A, Modolo G, Espartero AG, De Mendoza J (2004) PARTNEW—new solvent extraction process for minor actinides; CEA-R-6066. Commissariat à l’Energie Atomique, Paris
Geist A, Hill C, Modolo G, Foreman MRS, Weigl M, Gompper K, Hudson MJ, Madic C (2006) 6,6’-Bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[1,2,4]triazin-3-yl)[2,2’]bipyridine, an effective extracting agent for the separation of americium(III) and curium(III) from the lanthanides. Solvent Extr Ion Exch 24:463–483
Trumm S, Geist A, Panak P, Fanghänel T (2011) An improved hydrolytically-stable bis-triazinyl-pyridine (BTP) for selective actinide extraction. Solvent Extr Ion Exch 29:213–229
Ning S, Zou Q, Wang X, Liu R, Wei Y, Zhao Y, Ding Y (2016) Evalution study on silica/polymer-based CA-BTP adsorbent for the separation of minor actinides from simulated high-level liquid wastes. J Radioanal Nucl Chem 307:993–999
Horwitz EP, Dietz ML, Nelson DM, LaRosa JJ, Fairman WD (1990) Concentration and separation of actinides from urine using a supported bifunctional organophosphorus extractant. Anal Chim Acta 238:263–271
Horwitz EP, Dietz ML, Fisher DE (1991) Separation and preconcentration of strontium from biological, environmental, and nuclear waste samples by extraction chromatography using a crown ether. Anal Chem 63:522–525
Tai S, Williams NJ, Carrick JD (2016) Synthesis of bis-1,2,4-triazines via telescoped condensation of [1,10]-phenanthroline-2,9-dicarbonitrile with aromatic 1,2-dicarbonyls. J Heterocycl Chem 53:307–312
Hill TG, Chin AL, Tai S, Carrick JD, Ensor D, Delmau LH (2017) Separation of americium from europium using 3,3′-dimethoxy-phenyl-bis-1,2,4-triazinyl-2,6-pyridine. Sep Sci Technol. doi:10.1080/01496395.2017.1304419
Acknowledgements
This research was sponsored by the Office of Nuclear Energy, U.S. Department of Energy. One author (K.T.) would like to thank the Tennessee Technological University Student Research Grant Program for support. Thank you to Melissa Friedrich for her helpful edits.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tevepaugh, K.N., Coonce, J., Tai, S. et al. Chromatographic separation of americium from europium using bis-2,6-(5,6,7,8-tetrahydro-5,9,9-trimethyl-5,8-methano-1,2,4-benzotriazin-3-yl) pyridine. J Radioanal Nucl Chem 314, 371–376 (2017). https://doi.org/10.1007/s10967-017-5365-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5365-0