Abstract
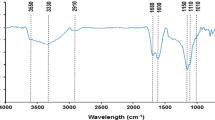
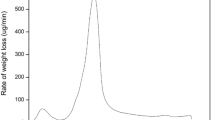
Uranium is the important nuclear fuel and its effective adsorption using low-cost materials is meaningful to environmental protection and sustainable development of nuclear energy. In this study, green and economical reed straw-derived biochar (RBC) was prepared by hydrothermal carbonization method using low-cost reed straw as feedstocks and citric acid as catalysts for uranium(VI) removal. RBC possessed larger pores and more oxygen-containing functional groups, providing more abundant adsorption sites for uranyl ions. RBC adsorbed uranium pH-dependently, spontaneously and endothermically, and pseudo-second-order kinetics and the Langmuir model could adequately describe the adsorption process. This work highlighted a feasible synthesis strategy of biochar for radioactive contaminants removal.






Similar content being viewed by others
References
Ye Q (2016) Safety and effective developing nuclear power to realize green and low-carbon development. Adv Clim Chang Res 7:10–16
Yan X, Luo X (2015) Radionuclides distribution, properties, and microbial diversity of soils in uranium mill tailings from southeastern China. J Environ Radioact 139:85–90
Papageorgiou F, McDermott F, Van Acken D (2022) Uranium in groundwaters: Insights from the Leinster granite, SE Ireland. Appl Geochem 139:105236
Gao Y, Yuan Y, Ma D, Li L, Li Y, Xu W, Tao W (2014) Removal of aqueous uranyl ions by magnetic functionalized carboxymethylcellulose and adsorption property investigation. J Nucl Mater 453:82–90
Yi Z, Yao J, Kuang Y, Chen H, Xu J (2016) Uptake of hexavalent uranium from aqueous solutions using coconut husk activated carbon. Desalin Water Treat 57:1749–1755
Zhao C, Liu J, Deng Y, Tian Y, Sun Q (2019) Uranium(VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J Clean Prod 236:117624
Dai Z, Zhen Y, Sun Y, Li L, Ding D (2021) ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(VI) removal. Chem Eng J 415:129002
Ahmad Z, Li Y, Yang J, Geng N, Fan Y, Gou X, Sun Q, Chen J (2022) A Membrane-Supported Bifunctional Poly(amidoxime-ethyleneimine) Network for Enhanced Uranium Extraction from Seawater and Wastewater. J Hazard Mater 425:127995
Yang X, Zhang Z, Kuang S, Wei H, Li Y, Wu G, Geng A, Li Y, Liao W (2020) Removal of thorium and uranium from leach solutions of ion-adsorption rare earth ores by solvent extraction with Cextrant 230. Hydrometallurgy 194:105343
Jun B-M, Lee H-K, Park S, Kim T-J (2021) Purification of uranium-contaminated radioactive water by adsorption: A review on adsorbent materials. Sep Purif Technol 278:119675
Wang Y, Wang Z, Gu Z, Yang J, Liao J, Yang Y, Liu N, Tang J (2015) Uranium(VI) sorption on graphene oxide nanoribbons derived from unzipping of multiwalled carbon nanotubes. J Radioanal Nucl Ch 304:1329–1337
Gu Z, Wang Y, Tang J, Yang J, Liao J, Yang Y, Liu N (2015) The removal of uranium(VI) from aqueous solution by graphene oxide–carbon nanotubes hybrid aerogels. J Radioanal Nucl Ch 303:1835–1842
Hu X, Wang Y, Wu P, Li Y, Tu H, Wang C, Yuan D, Liu Y, Cao X, Liu Z (2020) Preparation of graphene/graphene nanoribbons hybrid aerogel and its application for the removal of uranium from aqueous solutions. J Radioanal Nucl Ch 325:207–215
Hu X, Wang Y, Yang JO, Li Y, Wu P, Zhang H, Yuan D, Liu Y, Wu Z, Liu Z (2020) Synthesis of graphene oxide nanoribbons/chitosan composite membranes for the removal of uranium from aqueous solutions. Front Chem Sci Eng 14:1029–1038
Qiu B, Tao X, Wang H, Li W, Ding X, Chu H (2021) Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J Anal Appl Pyrol 155:105081
Huang Q, Song S, Chen Z, Hu B, Chen J, Wang X (2019) Biochar-based materials and their applications in removal of organic contaminants from wastewater: state-of-the-art review. Biochar 1:45–73
Tan X-F, Zhu S-S, Wang R-P, Chen Y-D, Show P-L, Zhang F-F, Ho S-H (2021) Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin Chem Lett 32:2939–2946
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sust Energ Rev 45:359–378
Tu W, Liu Y, Xie Z, Chen M, Ma L, Du G, Zhu M (2021) A novel activation-hydrochar via hydrothermal carbonization and KOH activation of sewage sludge and coconut shell for biomass wastes: Preparation, characterization and adsorption properties. J Colloid Interface Sci 593:390–407
Erses Yay AS, Birinci B, Açıkalın S, Yay K (2021) Hydrothermal carbonization of olive pomace and determining the environmental impacts of post-process products. J Clean Prod 315:128087
Chen C, Liu G, An Q, Lin L, Shang Y, Wan C (2020) From wasted sludge to valuable biochar by low temperature hydrothermal carbonization treatment: Insight into the surface characteristics. J Clean Prod 263:121600
Zhou N, Chen H, Xi J, Yao D, Zhou Z, Tian Y, Lu X (2017) Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour Technol 232:204–210
Fitri Faradilla RH, Lucia L, Hakovirta M (2021) Hydrothermal carbonization of soybean hulls for the generation of hydrochar: A promising valorization pathway for low value biomass. Environ Nanotechnol Monit Manage 16:100571
Ahmed W, Mehmood S, Qaswar M, Ali S, Khan ZH, Ying H, Chen D-Y, Núñez-Delgado A (2021) Oxidized biochar obtained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J Environ Chem Eng 9:105104
Paschalidou P, Pashalidis I, Manariotis ID, Karapanagioti HK (2020) Hyper sorption capacity of raw and oxidized biochars from various feedstocks for U(VI). J Environ Chem Eng 8:103932
Jin J, Li S, Peng X, Liu W, Zhang C, Yang Y, Han L, Du Z, Sun K, Wang X (2018) HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour Technol 256:247–253
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) An Assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manage 92:2504–2512
Li M, Li W, Liu S (2011) Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr Res 346:999–1004
Yang B, Liu Y, Liang Q, Chen M, Ma L, Li L, Liu Q, Tu W, Lan D, Chen Y (2019) Evaluation of activated carbon synthesized by one-stage and two-stage co-pyrolysis from sludge and coconut shell. Ecotoxicol Environ Saf 170:722–731
Hyun SP, Davis JA, Sun K, Hayes KF (2012) Uranium(VI) reduction by iron(II) monosulfide mackinawite. Environ Sci Technol 46:3369–3376
Ye T, Huang B, Wang Y, Zhou L, Liu Z (2020) Rapid removal of uranium(VI) using functionalized luffa rattan biochar from aqueous solution. Colloid Surf A 606:125480
Hadjittofi L, Pashalidis I (2015) Uranium sorption from aqueous solutions by activated biochar fibres investigated by FTIR spectroscopy and batch experiments. J Radioanal Nucl Ch 304:897–904
Yu X, Liu Y, Zhou Z, Xiong G, Cao X, Li M, Zhang Z (2014) Adsorptive removal of U(VI) from aqueous solution by hydrothermal carbon spheres with phosphate group. J Radioanal Nucl Ch 300:1235–1244
Atia BM, Khawassek YM, Hussein GM, Gado MA, El-Sheify MA, Cheira MF (2021) One-pot synthesis of pyridine dicarboxamide derivative and its application for uranium separation from acidic medium. J ENVIRON CHEM ENG 9:105726
Sanyal K, Chappa S, Pathak N, Pandey AK, Misra NL (2018) Trace element determinations in uranium by Total reflection X-Ray Fluorescence spectrometry using a newly developed polymer resin for major matrix separation. Spectrochim Acta B 150:18–25
Solouk A, Mirzadeh H, Shokrgozar MA, Solati-Hashjin M, Najarian S, Seifalian AM (2011) The study of collagen immobilization on a novel nanocomposite to enhance cell adhesion and growth. Iran Biomed J 15:6–14
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21966005, 22166001, 22066002) and Open Project Foundation of Nuclear Technology Application Ministry of Education Engineering Research Center (East China University of Technology) (HJSJYB2020-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, Y., Xia, H. et al. Low-cost reed straw-derived biochar prepared by hydrothermal carbonization for the removal of uranium(VI) from aqueous solution. J Radioanal Nucl Chem 331, 3915–3925 (2022). https://doi.org/10.1007/s10967-022-08421-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08421-y