Abstract
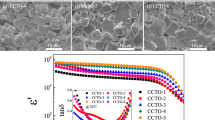
Calcium silicate (CaSiO3), wollastonite, with a molar ratio of CaO:SiO2 of 1:1, was synthesized by a sol–gel process and sintered at 1,100°C for 1 h. The synthesis of calcium silicate was carried out using chicken eggshells as the starting material possessing several advantages such as low cost, high purity, and less moisture sensitivity, when compared with those obtained from metal alkoxide precursors via the sol–gel process. The CaSiO3 samples have the triclinic or anorthic phase formations and good electrical properties. The dielectric constant and electrical conductivity are 62.59 ± 0.44 and 8.0052 × 10−4 (Ω.m)−1, respectively, at 25°C and 1 MHz. The transmission electron microscopy (TEM) images of the samples show a good dispersion and uniform particles with an average diameter of about 0.5 nm. X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Scanning electron microscopy (SEM), and Simultaneous thermal analysis (STA) were used to verify the synthesis.









Similar content being viewed by others
References
Chen XD, Freeman Y, Guo F, Chen P (1999) Diffusion of sodium chloride throughout chicken eggshell in relation to an ancient method of egg preservation. Trans IChemeE Part C 77:40–46
Davis C, Reeves R (2002) A report for the rural industries Research & Coorporation pp. 69
Thapon JL, Bourgeois CM (1994) Lavousier technique et documentation. L’Oeuf et les ovoproducts pp.334
Rivera EM, Araiza M, Brostow W, Castano VM, Díaz-Estrada JR, Hernández R, Rodríguez JR (1999) Synthesis of hydroxyapatite from eggshells. Mater Lett 41:128–134
Xia W, Chang J (2008) Preparation and the phase transformation behavior of amorphous mesoporous calcium silicate. Micropor Mesopore Mat 108:345–351
De Aza PN, Luklinsk ZB, Anseau MR, Hector M, Guitian F, De Aza S (2000) Reactivity of a wollastonite-tricalcium phosphate Bioeutectic® ceramic in human parotid saliva. Biomaterials 21:1735–1741
Deer WA, Howie RA, Zussman J (1963) Chain Silicate. Longmans. Green and Co. Ltd., London, p 167
Henmi C, Kawahara A, Henmi K (1983) The 37, 4T and 5Z polytypes of wollastonite from Kushiro, Hiroshima Prefecture. Am Minerol 68:156–163
Shieh SR, Duffy TS, Shen G (2004) Elasticity and strength of calcium silicate perovskite at lower mantle pressures. Phys Earth Planet In 143–144:93–105
Hayashi T, Saito H (1980) Preparation of CaO-SiO2 glasses by the gel method. J Mater Sci 15:1971–1977
Bansal NP (1990) Influence of Several Metal Ions on the Gelation Activation Energy of Silicon Tetraethoxide. J Am Ceram Soc 73:2647–2652
Iimori Y, Kameshima Y, Okada K, Hayashi S (2005) comparative study of apatite formation on CaSiO3 ceramics in simulated body fluids with different carbonate concentrations. J Mater Sci 16:73–79
Wang H, Zhang Q, Yang H, Sun H (2008) Synthesis and microwave dielectric properties of CaSiO3 nanopowder by the sol–gel process. Ceram Int 34:1405–1408
Wan X, Chang C, Mao D, Jiang L, Li M (2005) Preparation and in vitro bioactivities of calcium silicate nanophase materials. Mater Sci Eng C 25:455–461
Guinier A (1984) Nomenclature of polytype structures. Acta Cryst 40:399–404
Nickel EH (1993) Standardisation of polytype suffixes. Eur J Mineral 5:799–800
Acknowledgments
The authors would like to thank the following: The Petroleum and Petrochemical College and the Scientific and Technological Research Equipment Centre of Chulalongkorn University; and, the Departments of Materials Engineering and Physics, at Kasetsart University for the use of the analytical equipment. We are also grateful for the grant support from the Kasetsart University Research and Development Fund, fiscal year 2009. AS would like to acknowledge the financial supports from the Conductive and Electroactive Polymers Research Unit, the Thailand Research Fund (TRF-BRG), and the Royal Thai Government (Budget of Fiscal Year 2552).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tangboriboon, N., Khongnakhon, T., Kittikul, S. et al. An innovative CaSiO3 dielectric material from eggshells by sol–gel process. J Sol-Gel Sci Technol 58, 33–41 (2011). https://doi.org/10.1007/s10971-010-2351-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-010-2351-1