Abstract
All-solid-state batteries are an attractive proposal to achieve the demands for safe, efficient and high energy storage. This paper briefly discusses the current challenges in the field of all-solid-state batteries and how sol-gel routes can addressed them. The application of the sol-gel process for the synthesis of solid electrolytes is described, with special emphasis on solid oxide-type electrolytes. In this context, the use of sol-gel derived sintering additives is discussed. The sol-gel process involved in the fabrication of oxide-type all-solid-state battery is also described. Chemical strategies based on sol-gel technology to prepare cathode|electrolyte and anode-lithium metal|electrolyte interfaces with low interfacial resistance are described and contrasted with state-of-art literature.

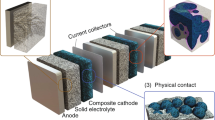
Graphical abstract
Highlights
-
Sol-gel process used to prepare oxide-type all-solid-state batteries.
-
Sol-gel process for the synthesis of solid electrolytes, polymeric and inorganic electrolytes.
-
Sol-gel sintering additives used for the synthesis of solid electrolytes.
-
Sol-gel derived interfacial materials and buffer layers for low interfacial electrode/electrolyte resistance.







Similar content being viewed by others
References
© European Union, Towards clean, competitive and connected mobility: the contribution of Transport. Research and Innovation to the Mobility package. 2017. p. SWD 223.
Kim KJ et al. (2021) Solid-state Li–metal batteries: Challenges and horizons of oxide and sulfide solid electrolytes and their interfaces. Adv Energy Mater 11(1):2002689
Kato Y, Hori S, Kanno R (2020) Li10GeP2S12-type superionic conductors: Synthesis, structure, and ionic transportation. Adv Energy Mater 10(42):2002153
Zhu Y, He X, Mo Y (2015) Origin of outstanding stability in the lithium solid electrolyte materials: Insights from thermodynamic analyses based on first-principles calculations. ACS Appl Mater Interfaces 7(42):23685–23693
Wang Q et al. (2019) Siloxane-based polymer electrolytes for solid-state lithium batteries. Energy Storage Mater 23:466–490
Klein L, Aparicio M, Damay F (2018) Sol-Gel Processing for Battery and Fuel Cell Applications. In: Klein L, Aparicio M, Jitianu A Eds Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications. Springer International Publishing, Cham, p 2573–2593
Rosero-Navarro NC, Tadanaga K (2018) Sol-Gel Processing of Solid Electrolytes for Li-Ion Batteries. In: Klein L, Aparicio M, Jitianu A Eds Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications. Springer International Publishing, Cham, p 2631–2648
Karabelli D, Birke KP, Weeber M (2021) A performance and cost overview of selected solid-state electrolytes: Race between polymer electrolytes and inorganic sulfide electrolytes. Batteries 7(1):18
Zhao Q et al. (2020) Designing solid-state electrolytes for safe, energy-dense batteries. Nat Rev Mater 5(3):229–252
Feng J et al. (2021) PEO based polymer-ceramic hybrid solid electrolytes: a review. Nano Convergence 8(1):2
Ngai KS et al. (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics 22(8):1259–1279
Armand, M.B., et al. Polymer Electrolytes, in Energy Materials. 2011. p. 1–31.
Zelazowska, E., E. Rysiakiewicz-Pasek, and M. Borczuch-LŁaczka. Sol-gel derived Li-ion conducting polymer electrolytes. in Materials Science- Poland. 2005.
Liang H-P et al. (2022) Polysiloxane-based single-ion conducting polymer blend electrolyte comprising small-molecule organic carbonates for high-energy and high-power lithium-metal batteries. Adv Energy Mater 12(16):2200013
Meng N, Zhu X, Lian F (2022) Particles in composite polymer electrolyte for solid-state lithium batteries: A review. Particuology 60:14–36
Hayashi A et al. (2001) Preparation of Li2S–P2S5 amorphous solid electrolytes by mechanical milling. J Am Ceram Soc 84(2):477–79
Hayashi A et al. (2003) Formation of superionic crystals from mechanically milled Li2S–P2S5 glasses. Electrochem Commun 5(2):111–114
Mizuno F et al. (2005) New, highly ion-conductive crystals precipitated from Li2S–P2S5 glasses. Adv Mater 17(7):918–921
Tatsumisago M, Hayashi A (2014) Sulfide glass-ceramic electrolytes for all-solid-state lithium and sodium batteries. Int J Appl Glass Sci 5(3):226–235
Kamaya N et al. (2011) A lithium superionic conductor. Nat Mater 10(9):682–6
Kato Y et al. (2016) High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy 1:16030
Murugan R, Thangadurai V, Weppner W (2007) Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem 46(41):7778–81
Tadanaga K et al. (2013) Low temperature synthesis of highly ion conductive Li7La3Zr2O12–Li3BO3 composites. Electrochem Commun 33:51–54
Samson AJ et al. (2019) A bird’s-eye view of Li-stuffed garnet-type Li7La3Zr2O12 ceramic electrolytes for advanced all-solid-state Li batteries. Energy Environ Sci 12(10):2957–2975
Wang C et al. (2020) Garnet-type solid-state electrolytes: Materials, interfaces, and batteries. Chem Rev 120(10):4257–4300
Subramanian K et al. (2021) A brief review of recent advances in garnet structured solid electrolyte based lithium metal batteries. J Energy Storage 33:102157
Rosero-Navarro NC, Tadanaga K (2019) Sintering Additives for Garnet-Type Electrolytes. In: Murugan R, Weppner W Eds Solid Electrolytes for Advanced Applications: Garnets and Competitors. Springer International Publishing, Cham, p 111–128
Ohta S et al. (2014) Co-sinterable lithium garnet-type oxide electrolyte with cathode for all-solid-state lithium ion battery. J Power Sources 265(0):40–44
Janani N et al. (2015) Optimization of lithium content and sintering aid for maximized Li+ conductivity and density in Ta-Doped Li7La3Zr2O12. J Am Ceram Soc 98(7):2039–2046
Rosero-Navarro NC et al. (2017) Optimization of Al2O3 and Li3BO3 content as sintering additives of Li7−x La2.95Ca0.05ZrTaO12 at low temperature. J Electron Mater 46(1):497–501
Jonson RA, McGinn PJ (2018) Tape casting and sintering of Li7La3Zr1.75Nb0.25Al0.1O12 with Li3BO3 additions. Solid State Ion 323:49–55
Xie H et al. (2019) Consolidating the grain boundary of the garnet electrolyte LLZTO with Li3BO3 for high-performance LiNi0.8Co0.1Mn0.1O2/LiFePO4 hybrid solid batteries. J Mater Chem A 7(36):20633–20639
Han J, Kim JC (2020) A solid-state route to stabilize cubic Li7La3Zr2O12 at low temperature for all-solid-state-battery applications. Chem Commun 56(96):15197–15200
Rosero-Navarro NC, Tadanaga K (2016) Sol–Gel Processing of Solid Electrolytes for Li-ion Batteries. In: Klein L, Aparicio M, Jitianu A Eds Handbook of Sol-Gel Science and Technology. Springer International Publishing, Cham, p 1–18
Rosero-Navarro NC et al. (2021) Synthesis of highly Li-ion conductive garnet-type solid ceramic electrolytes by solution-process-derived sintering additives. J Eur Ceram Soc 41(13):6767–6771
Hongahally Basappa R et al. (2017) Grain boundary modification to suppress lithium penetration through garnet-type solid electrolyte. J Power Sources 363:145–152
Xu B et al. (2017) Li3PO4-added garnet-type Li6.5La3Zr1.5Ta0.5O12 for Li-dendrite suppression. J Power Sources 354:68–73
Zheng C et al. (2021) Grain boundary modification in garnet electrolyte to suppress lithium dendrite growth. Chem Eng J 411:128508
Liu Q et al. (2018) Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J Power Sources 389:120–134
Ramakumar S et al. (2017) Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog Mater Sci 88:325–411
Bucci G et al. (2017) Modeling of internal mechanical failure of all-solidstate batteries during electrochemical cycling, and implications for battery design. J Mater Chem A 5(36):19422–19430
Cheng L et al. (2014) The origin of high electrolyte–electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys Chem Chem Phys 16(34):18294–18300
Sharafi A et al. (2017) Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state Electrolyte Li7La3Zr2O12. Chem Mater 29(18):7961–7968
Li Y et al. (2018) Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J Am Chem Soc 140(20):6448–6455
Basappa RH, Ito T, Yamada H (2017) Contact between Garnet-type solid electrolyte and lithium metal anode: Influence on charge transfer resistance and short circuit prevention. J Electrochem Soc 164(4):A666–A671
Ohta S, Kobayashi T, Asaoka T (2011) High lithium ionic conductivity in the garnet-type oxide Li7−X La3(Zr2−X, NbX)O12 (X=0–2). J Power Sources 196(6):3342–3345
Ohta S et al. (2012) Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte. J Power Sources 202(0):332–335
Kato T et al. (2014) In-situ Li7La3Zr2O12/LiCoO2 interface modification for advanced all-solid-state battery. J Power Sources 260:292–298
Matsuyama T et al. (2016) Fabrication of all-solid-state lithium secondary batteries with amorphous TiS4 positive electrodes and Li7La3Zr2O12 solid electrolytes. Solid State Ion 285:122–125
Kotobuki M et al. (2010) Compatibility of Li7La3Zr2O12 solid electrolyte to all-solid-state battery using Li metal anode. J Electrochem Soc 157(10):A1076–A1079
Ohta S et al. (2013) All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J Power Sources 238(0):53–56
Liu T et al. (2016) Achieving high capacity in bulk-type solid-state lithium ion battery based on Li6.75La3Zr1.75Ta0.25O12 electrolyte: Interfacial resistance. J Power Sources 324:349–357
Han F et al. (2018) Interphase engineering enabled all-ceramic lithium battery. Joule 2(3):497–508
Alexander GV et al. (2018) Electrochemical performance of a garnet solid electrolyte based lithium metal battery with interface modification. J Mater Chem A 6(42):21018–21028
Jin Y, McGinn PJ (2013) Bulk solid state rechargeable lithium ion battery fabrication with Al-doped Li7La3Zr2O12 electrolyte and Cu0.1V2O5 cathode. Electrochim Acta 89:407–412
Rosero-Navarro NC et al. (2020) Significant reduction in the interfacial resistance of garnet-type solid electrolyte and lithium metal by a thick amorphous lithium silicate layer. ACS Appl Energy Mater 3(6):5533–5541
Han X et al. (2016) Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat Mater 16:572
Rosero-Navarro NC et al. (2020) Organic–inorganic hybrid materials for interface design in all-solid-state batteries with a garnet-type solid electrolyte. ACS Appl Energy Mater 3(11):11260–11268
Xue Z, He D, Xie X (2015) Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A 3(38):19218–19253
Vélez JF, Aparicio M, Mosa J (2016) Covalent silica-PEO-LiTFSI hybrid solid electrolytes via sol-gel for Li-ion battery applications. Electrochim Acta 213:831–841
Zhang J et al. (2016) Solid polymer electrolyte membranes based on organic/inorganic nanocomposites with star-shaped structure for high performance lithium ion battery. J Membr Sci 509:138–148
Boaretto N et al. (2017) Optimization of the transport and mechanical properties of polysiloxane/polyether hybrid polymer electrolytes. Electrochim Acta 241:477–486
Nakano S et al. (2018) Effect of chain architectures of star-shaped Poly(ethylene glycol) macromonomers on enhancement of thermal, mechanical, and electrochemical performance of polymer electrolyte membranes. Chem Lett 47(4):587–590
Zhang J et al. (2018) A star-shaped solid composite electrolyte containing multifunctional moieties with enhanced electrochemical properties for all solid-state lithium batteries. J Membr Sci 552:107–114
Schnell J et al. (2018) All-solid-state lithium-ion and lithium metal batteries–paving the way to large-scale production. J Power Sources 382:160–175
Han F et al. (2019) High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat Energy 4(3):187–196
Cheng EJ, Sharafi A, Sakamoto J (2017) Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim Acta 223:85–91
Krauskopf T et al. (2019) Lithium-metal growth kinetics on LLZO Garnet-type solid electrolytes. Joule 3(8):2030–2049
Kim S et al. (2020) The role of interlayer chemistry in Li-metal growth through a Garnet-type solid electrolyte. Adv Energy Mater 10(12):1903993
Luo W et al. (2016) Transition from superlithiophobicity to superlithiophilicity of garnet solid-state electrolyte. J Am Chem Soc 138(37):12258–12262
Luo W et al. (2017) Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer. Adv Mater 29(22):1606042
Wang C et al. (2017) Conformal, Nanoscale ZnO –surface modification of garnet-based solid-state electrolyte for lithium metal anodes. Nano Lett 17(1):565–571
Fu K et al. (2017) Toward garnet electrolyte–based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci Adv 3(4):e1601659
Fu K et al. (2017) Transient behavior of the metal interface in lithium metal–garnet batteries. Angew Chem Int Ed 56(47):14942–14947
Alexander GV et al. (2018) Electrodes-electrolyte interfacial engineering for realizing room temperature lithium metal battery based on garnet structured solid fast Li+ conductors. J Power Sources 396:764–773
He M et al. (2018) Formation of self-limited, stable and conductive interfaces between garnet electrolytes and lithium anodes for reversible lithium cycling in solid-state batteries. J Mater Chem A 6(24):11463–11470
Lu Y et al. (2018) An in situ element permeation constructed high endurance Li–LLZO interface at high current densities. J Mater Chem A 6(39):18853–18858
Acknowledgements
The author acknowledges the financial support: Nippon Sheet Glass Foundation FY2021, KAKENHI 17K17559 by Japan Society for Promotion of Science and SOUSEI Program and Young FS Project 2017 by Hokkaido University. The author thanks to her major collaborators in this topic, Prof. Kiyoharu Tadanaga and Prof. Akira Miura of Hokkaido University. Dr. Randy Jalem and Prof. Yoshitaka Tateyama of National Institute for Materials Science are recognized for their collaboration in computational calculations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosero-Navarro, N.C. Application of sol-gel processes to materials and interfaces in oxide-based all-solid-state batteries. J Sol-Gel Sci Technol 103, 680–689 (2022). https://doi.org/10.1007/s10971-022-05880-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05880-3