Abstract
In our previous work, we adopted the organic acids assisted sol–gel method to prepare ZrO2 aerogels. In preparation process, we found the organic acids with two carboxyl groups and one side group including –OH, –NH2, and –SH were prerequisite to form wet gels. Furthermore, the citric acid with three carboxyl groups and one hydroxyl group was also successfully to form wet gels. Therefore, we speculated that the organic acids required to possess at least two carboxyl groups and one side group to form wet gels. In this work, different organic reagents including acetic acid (AA), oxalic acid (OA), lactic acid (LA), edetic acid (EDTA), butanediol (BD), glycerol (GA), citric acid (CA), L-malic acid (LMA) and L-tartaric acid (LTA) were adopted as gel accelerators to verify our assumption. A series of ZrO2 aerogels were successfully synthesized using ZrOCl2·8H2O as zirconium precursor, and LMA or LTA as the gel initiators by the sol–gel method. It was found that the gelation time of LTA as gelator was shorted than that of LMA in the same condition. When LTA as an gelator, the tetragonal phase can keep until 1000 °C, while for LMA, the tetragonal phase can keep at 800 °C. After supercritical fluid drying (SCFD), ZrO2 aerogel with high surface area of over 339 m2·g−1 and large pore volume of over 0.92 cm3·g−1 can be obtained.
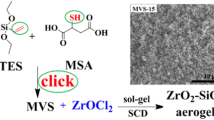
Graphical Abstract

ZrO2 aerogels have been prepared applied L-malic acid or L-tartaric acid as gelators by sol–gel method.
Highlights
-
ZrO2 aerogels have been prepared applying the zirconium source (ZrOCl2·8H2O) and L-malic acid or, L-tartaric acid.
-
The organic acids at least had one side group –OH and two –COOH groups, which were integrant to synthesize ZrO2 aerogel.
-
LMA-6-aerogel has high surface area over 339 m2·g−1 and large pore volume over 0.92 cm3·g−1.





Similar content being viewed by others
References
Rebecca CW, Anna EP, Andres PH, James KF (2021) Zirconia aerogels for thermal management: Review of synthesis, processing, and properties information architecture. Adv Colloid Interface Sci 295:102464
Koebel M, Rigacci A, Achard P (2012) Aerogel-based thermal superinsulation: an overview. J Sol-Gel Sci Techn 63:315–339
Xu X, Zhang Q, Hao M, Hu Y, Lin Z, Peng L, Wang T, Ren X, Wang C, Zhao Z, Wan C, Fei H, Wang L, Zhu J, Sun H, Chen W, Du T, Deng B, Cheng GJ, Shakir I, Dames C, Fisher TS, Zhang X, Li H, Huang Y, Duan X (2019) Double-negative-index ceramic aerogels for thermal superinsulation. Science 363:723–727
Pierre AC, Pajonk GM (2002) Chemistry of Aerogels and Their Applications. Chem Rev 102:4243–4266
Yu L, Yao L, Yang K (2016) Redox- and pH-responsive hydrogels: formulation and controlled drug delivery. J Porous Mat 23:1581–1589
Štandeker S, Novak Z, Knez Ž (2007) Adsorption of toxic organic compounds from water with hydrophobic silica aerogels. J Colloid Interface Sci 310:362–368
Wang ML, Liu BL, Ren CC, Shih ZW (1997) Preparation of the Precursor of the Zirconium Oxide in EDTA−Ammonia Solution by the Sol−Gel Method. Ind Eng Chem Res 36:2149–2155
Olson NS, Hurwitz FI, Guo H, Madden NJ, Stokes JL, Rogers RB, Krogstad JA (2021) Enhanced thermal stability of high yttria concentration YSZ aerogels. J Am Ceram Soc 104:4190–4202
Yu H, Tong Z, Qiao Y, Yang Z, Yue S, Li X, Su D, Ji H (2020) High thermal stability of SiO2–ZrO2 aerogels using solvent-thermal aging. J Solid State Chem 291:121624
Alwin S, Sahaya Shajan X (2020) Aerogels: promising nanostructured materials for energy conversion and storage applications. Mater Renew Sustain Energy 9:7
Esquivel-Castro TA, Martínez-Luévanos A, Cabrera AR, García-Cerda LA, Esparza-González SC, Ibarra-Alonso MC, Estrada-Flores S (2022) ZrO2 aerogels as drugs delivery platforms: Synthesis, cytotoxicity, and diclofenac delivery. J Drug Deliv Sci Tech 77:103837
Rad M, Borhani S, Moradi M, Safarifard V (2021) Tuning the crystallinity of ZrO2 nanostructures derived from thermolysis of Zr-based aspartic acid/succinic acid MOFs for energy storage application. Phys E 134:114921
Carsten S, Alfons B (1998) Zirconia aerogels: effect of acid-to-alkoxide ratio, alcoholic solvent and supercritical drying method on structural properties. J Non-Cryst Solids 223:165–178
He J, Li XL, Su D, Ji HM, Zhang X, Zhang WS (2016) Super-hydrophobic hexamethyl-disilazane modified ZrO2–SiO2 aerogels with excellent thermal stability. J Mat Chem: A 4:5632–5638
Wang QP, Li XL, Fen WP, Ji HM, Sun XH, Xiong R (2014) Properties of electrodeposited Ni–B–Al2O3 composite coatings. J Porous Mat 21:127–130
Schäfer H, Brandt S, Milow B, Ichilmann S, Steinhart M, Ratke L (2013) Zirconia-based aerogels via hydrolysis of salts and alkoxides: the influence of the synthesis procedures on the properties of the aerogels. Chem Asian J 8:2211–2219
Southon PD, Bartlett JR, Woolfrey JL, Ben-Nissan B (2002) Formation and Characterization of an Aqueous Zirconium Hydroxide Colloid. Chem Mat 14:4313–4319
Torres-Rodríguez J, Kalmár J, Menelaou M, Čelko L, Dvořak K, Cihlář Jr J, Cihlář J, Kaiser J, Győri E, Veres P, Fábián I, Lázár I (2019) Heat treatment induced phase transformations in zirconia and yttria-stabilized zirconia monolithic aerogels. J Supercrit Fluid 149:54–63
Gossard A, Toquer G, Grandjean S, Grandjean A (2014) Coupling between SAXS and Raman spectroscopy applied to the gelation of colloidal zirconium oxy-hydroxide systems J Sol-Gel Sci Techn 71:571–579
Schafer H, Milow B, Ratke L (2013) Synthesis of inorganic aerogels via rapid gelation using chloride precursors. RSC Adv 3:15263–15272
Wang X, Li C, Shi Z, Zhi M, Hong Z (2018) The investigation of an organic acid assisted sol-gel method for preparing monolithic zirconia aerogels. RSC Adv 8:8011–8020
Wang X, Wu Z, Zhi M, Hong Z (2018) Synthesis of high temperature resistant ZrO2-SiO2 composite aerogels via “thiol-ene” click reaction. J Sol-Gel Sci Techn 87:734–742
Zhang Z, Gao Q, Gao H, Shi Z, Wu J, Zhi M, Hong Z (2016) Nickel oxide aerogel for high performance supercapacitor electrodes. RSC Adv 6:112620–112624
Zhang Z, Gao Q, Liu Y, Zhou C, Zhi M, Hong Z, Zhang F, Liu B (2015) A facile citric acid assisted sol-gel method for preparing monolithic yttria-stabilized zirconia aerogel. RSC Adv 5:84280–84283
Gao Q, Wang X, Shi Z, Ye Z, Wang W, Zhang N, Hong Z, Zhi M (2018) Synthesis of porous NiCo2S4 aerogel for supercapacitor electrode and oxygen evolution reaction electrocatalyst. Chem Eng J 331:185–193
Chao X, Yuan W, Shi Q, Zhu Z (2016) Improvement of thermal stability of zirconia aerogel by addition of yttrium. J Sol-Gel Sci Techn 80:667–674
Acknowledgements
This work is supported by National key research and development program (Grant No. 2016YFB0901600), National Nature Science Foundation of China (Grant No. 21303162 and Grant No. 11604295), the school fund (Grant No. 2020qd10), and General Natural Science Research Projects of Colleges and Universities in Anhui Province (Grant No. KJ2021B14), Key Research and Development Program of Anhui Province (Grant No. 202104b11020010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Li, C., Zhi, M. et al. Preparation of ZrO2 aerogels by L-malic acid and L-tartaric acid assistant sol–gel method. J Sol-Gel Sci Technol 106, 281–287 (2023). https://doi.org/10.1007/s10971-023-06067-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06067-0