Abstract
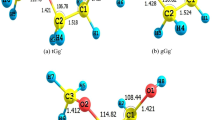
The energetic study of 6-methyl-1-indanone, 6-methoxy-1-indanone and 5,6-dimethoxy-1-indanone has been developed using calorimetric techniques and a computational methodology. The enthalpies of combustion and of sublimation of these compounds were determined from, respectively, static-bomb combustion calorimetry and high-temperature Calvet microcalorimetry. From these experimental data, the gas-phase standard molar enthalpies of formation were derived. Also, the temperature and the enthalpy of fusion of each compound were obtained by differential scanning calorimetry. Additionally, the gas-phase standard molar enthalpies of formation of these compounds were obtained from high-level ab initio calculations, at the G3(MP2)//B3LYP level of theory. The computational approach of these three indanone derivatives allowed us to establish their molecular structures, the co-existence of two and four stable conformations for 6-methoxy-1-indanone and 5,6-dimethoxy-1-indanone, respectively. Furthermore, the energetic effects associated with the presence of one methyl group and one or two methoxy groups in the indanone structure were evaluated. These enthalpic increments were compared with the homologous substitutions in the benzene and naphthalene molecules.




Similar content being viewed by others
References
Kohl T, Sapei E, Rocha IM, Galvao TL, Ribeiro da Silva MDMC, Ribeiro da Silva MAV. Modeling of fast pyrolysis of wood for prediction of bio-oil composition. In: Asia pacific confederation of chemical engineering congress 2015: APCChE 2015, incorporating CHEMECA. Engineers Australia; 2015. P. 2410.
Rocha IM, Galvão TLP, Sapei E, Ribeiro da Silva MDMC, Ribeiro da Silva MAV. Levoglucosan: a calorimetric, thermodynamic, spectroscopic, and computational investigation. J Chem Eng Data. 2013;58:1813–21.
Freitas VLS, Lima ACMO, Sapei E, Ribeiro da Silva MDMC. Comprehensive thermophysical and thermochemical studies of vanillyl alcohol. J Chem Thermodyn. 2016;102:287–92.
Sabbah R, Xu-wu A, Chickos JS, Leitão MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:193–204.
Gundry HA, Harrop D, Head AJ, Lewis GB. Thermodynamic properties of organic oxygen compounds 21. Enthalpies of combustion of benzoic acid, pentan-1-ol, octan-1-ol, and hexadecan-1-ol. J Chem Thermodyn. 1969;1:321–32.
Ribeiro da Silva MDMC, Santos LMNBF, Silva ALR, Fernandes O, Acree WE Jr. Energetics of 6-methoxyquinoline and 6-methoxyquinoline N-oxide: the dissociation enthalpy of the (N–O) bond. J Chem Thermodyn. 2003;35:1093–100.
Hubbard WN, Scott DW, Waddington G. Standard states and corrections for combustions in a bomb at constant volume. In: Rossini FD, editor. Experimental thermochemistry, vol. 1. New York: Interscience; 1956. p. 75–128.
Adedeji FA, Brown DLS, Connor JA, Leung WL, Paz-Andrade IM, Skinner HA. Thermochemistry of arene chromium tricarbonyls and the strenghts of arene-chromium bonds. J Organomet Chem. 1975;97:221–8.
Santos LMNBF, Schröder B, Fernandes OOP, Ribeiro da Silva MAV. Measurement of enthalpies of sublimation by drop method in a Calvet type calorimeter: design and test of a new system. Thermochim Acta. 2004;415:15–20.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JAJ, Stratmann RE, Burant JC, et al. Gaussian03, revision C.01. Wallingford: Gaussian Inc.; 2004.
Baboul AG, Curtiss LA, Redfern PC, Raghavachari K. Gaussian-3 theory using density functional geometries and zero-point energies. J Chem Phys. 1999;110:7650–7.
Rossini FD. Assignment of uncertainties to thermochemical data. In: Rossini FD, editor. Experimental thermochemistry, vol. 1. New York: Interscience; 1956. p. 297–325.
Olofson G. Assignment of uncertainties. In: Sunner S, Mansson M, editors. Combustion calorimetry, vol. 1. Oxford: Pergamon Press; 1979. p. 137–59.
Cox JD, Wagman DD, Medvedev VA. CODATA key values for thermodynamics. New York: Hemisphere; 1979.
Irikura KK, Thermo PL. National institute of standards and technology; 2002.
Merrick JP, Moran D, Radom L. An evaluation of harmonic vibrational frequency scale factors. J Phys Chem A. 2007;111:11683–700.
Roux MV, Temprado M, Chickos JS, Nagano Y. Critically evaluated thermochemical properties of polycyclic aromatic hydrocarbons. J Phys Chem Ref Data. 2008;37:1855–996.
Matos MAR, Miranda MS, Monte MJS, Santos LMNBF, Morais VMF, James S, Chickos JS, Umnahanant P, Liebman JF. Calorimetric and computational study of indanones. J Phys Chem A. 2007;111:11153–9.
Speros DM, Rossini FD. Heats of combustion and formation of naphthalene, the two methylnaphthalenes, cis and trans decahydronaphthalene and related compounds. J Phys Chem. 1960;64:1723–7.
Pedley JP. Thermochemical data and structures of organic compounds. College Station: Thermodynamics Research Centre; 1994.
Silva ALR, Freitas VLS, Ribeiro da Silva MDMC. Effects of methoxy and formyl substituents on the energetics and reactivity of α-naphthalenes: a calorimetric and computational study. Chemosphere. 2014;107:203–10.
Matos MAR, Miranda MS, Morais VMF. Calorimetric and theoretical determination of standard enthalpies of formation of dimethoxy- and trimethoxybenzene isomers. J Phys Chem A. 2000;104:9260–5.
Acknowledgements
Thanks are due to Fundação para a Ciência e Tecnologia (FCT), Lisbon, Portugal, for the financial support to Project UID/QUI/0081/2013 and to FEDER through Program NORTE2020 for the financial support to Project POCI-01-0145-FEDER-006980 and to Project “Sustained Advanced Materials,” ref. NORTE-01-0145-FEDER-000028 (FCUP).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, A.L.R., Lima, A.C.M.O. & Ribeiro da Silva, M.D.M.C. Energetic characterization of indanone derivatives involved in biomass degradation. J Therm Anal Calorim 134, 1267–1276 (2018). https://doi.org/10.1007/s10973-018-7533-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7533-z